Results for: "right-to-try"
Are antivaxers “holding science hostage”?
Melinda Wenner Moyer published an article in The New York Times arguing that fear of how antivaxers will react to scientific findings is leading scientists to self-censor. I'm not convinced that this is the case.

Medical science policy in the U.S. under Donald Trump eighty days in
A week after Donald Trump was elected, I speculated about how he would affect medical science policy. Now, 80 days into the Trump administration, we have some observations.

The Texas Medical Board lets Stanislaw Burzynski off lightly: A cautionary tale of the failure of regulating medicine
After three years and countless twists and turns, the final decision by the Texas Medical Board on the sanctions to be imposed on Houston cancer quack Dr. Stanislaw Burzynski were announced on Friday. Sadly, they were not enough. The Burzynski saga should serve as a cautionary tale that the regulation of physicians and medicine is too lax, not too strict.
Bills remove impediments to ill-advised state “right to try” laws, shield wrongdoers, and hide adverse events
Congressional bills will unleash state "right to try" laws, block terminally ill patients from redress for damages caused by negligent doctors and drug companies, and hide adverse drug events from the public.
Donald Trump versus the FDA: Is the standard of evidence for drug approval actually too low rather than too high?
All of the candidates being considered by President Trump for FDA Commissioner believe that the FDA is too strict in its standards for approving new drugs. In a commentary in Nature last week, two bioethicists argued that, at least in terms of preclinical data, the standard of evidence is actually too low. Which is correct?

Donald Trump and Peter Thiel vs. the FDA: Be afraid. Be very afraid.
Donald Trump's three most likely picks for FDA Commissioner all favor loosening drug approval standards. Two are cronies of Peter Thiel, of which one believes that the FDA shouldn't require evidence of efficacy, only safety, and the other believes that a "Yelp for drugs" would do a better job than the FDA. The third candidate is a bona fide, honest-to-goodness pharma shill....

Medical science policy in the U.S. under Donald Trump
The election of Donald Trump was unexpected. Given Trump's history of antivaccine beliefs and conspiracy theories, coupled with a fervor for deregulation (a fervor shared by the Republican Congress), it is reasonable to fear what will happen to medical science policy during the next four years.

FDA Looks At Dubious Stem Cell Clinics
Using stem cells to treat disease or improve recovery is an exciting area of research. The potential is undeniably great – these are cells that have the potential to differentiate into mature cells of a specific type. They can be used to replace damaged cells or improve the environment for cell function and recovery. Ideally stem cells can be developed from cells...
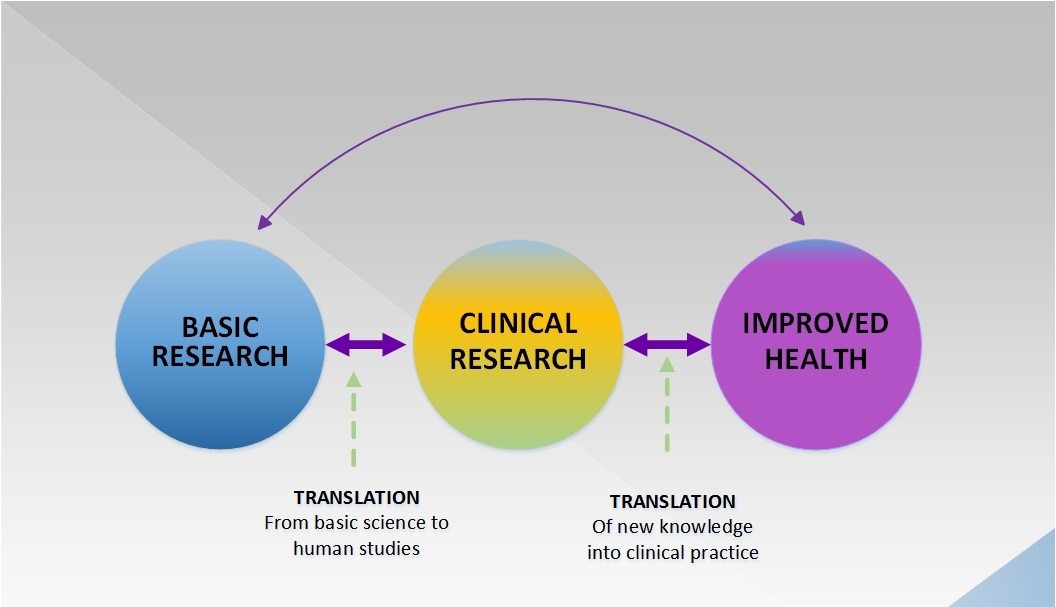
Whither the randomized controlled clinical trial?
With the rise of precision medicine and genomics, the conventional randomized clinical trial appears more and more outdated. Fortunately, clinical trials are evolving, but will it be enough to incorporate the numerous advances in "-omic" medicine in a rigorous scientific manner to benefit patients?
False balance about Stanislaw Burzynski and his disproven cancer therapy, courtesy of STAT News
One common theme that has been revisited time and time again on this blog since its very founding is the problem of how science and medicine are reported. For example, back when I first started blogging, years before I joined Science-Based Medicine in 2008, one thing that used to drive me absolutely nuts was the tendency of the press to include in...