Tag: Food and Drug Administration

FDA strengthens homeopathic drug enforcement (but falls short of actually enforcing the law)
The FDA may strengthen homeopathic drug regulation with its "risk-based" enforcement policy, but this still leaves illegal homeopathic remedies on the market and falls far short of actually enforcing the law.
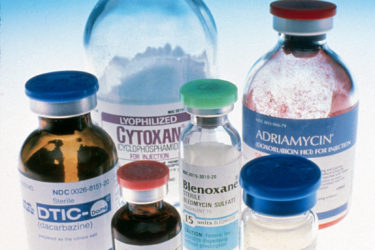
Drug shortages are worsening, and there are no simple solutions
Drug shortages, which worsen medical care and patient outcomes, are becoming more and more common. A new Task Force report from the FDA offers a potential way forward.

FDA proposes ban on curcumin and other naturopathic favorites in compounded drugs
Based on a thorough review of the evidence by experts, the FDA is proposing a ban on using curcumin, cesium chloride and other naturopathic favorites in compounded drugs.

Federal “right-to-try” over a year later: Still a failure and still about the money (and weakening the FDA)
Federal "right-to-try" legislation was passed and signed into law by President Trump over a year ago. Advocates promised that lots of terminally ill people who were dying then would be saved by having the right to "try" experimental therapies outside of the context of clinical trials. That has not happened. This should come as no surprise, because right-to-try was never about getting...

Generic Drugs and Global Deception
Generic drugs are supposed to be equivalent to brand-name drugs, but all too often they are defective. Katherine Eban's book shows that corrupt overseas manufacturers have committed intentional global fraud. The system is broken and the FDA lacks the power to correct abuses.
“Young blood” infusions: same old snake oil
There's no reliable evidence that an infusion of blood plasma from a young donor will benefit an older person, and there are risks, but Ambrosia Health is selling "young blood" infusions for thousands of dollars anyway. The FDA has taken notice.
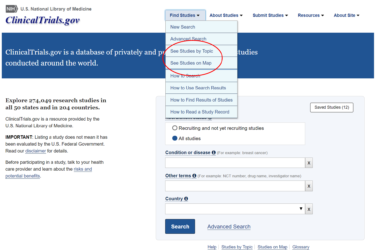
Dubious for-profit stem cell clinics: Co-opting ClinicalTrials.gov as a marketing tool
Over twenty years ago, cancer quack Stanislaw Burzynski pioneered the abuse of the clinical trial process as a marketing tool to sell his antineoplastons. Now, for-profit stem cell clinics are using ClinicalTrials.gov as a marketing tool for their unproven therapies by listing dubious and scientifically worthless trials in this government database. What can be done?

Patients Blinded by Stem Cell Therapy: FDA (and consumers) win a legal victory!
The Food and Drug Administration just won a court case supporting the agency's ability to regulate stem cell clinics that rely on client-derived adipose tissues. This is a win for consumer protection, though too late to help those already harmed.

Walmart sued for deceiving customers in selling homeopathic remedies
A lawsuit claiming Walmart fraudulently deceives consumers in the sale of worthless homeopathic remedies has been filed by the Center for Inquiry (CFI), acting on behalf of the general public. CFI says co-mingling ineffective homeopathic products with science-based treatments on Walmart's pharmacy shelves and website misleads customers into thinking they are equivalent, when "there is not a shred of credible scientific evidence"...
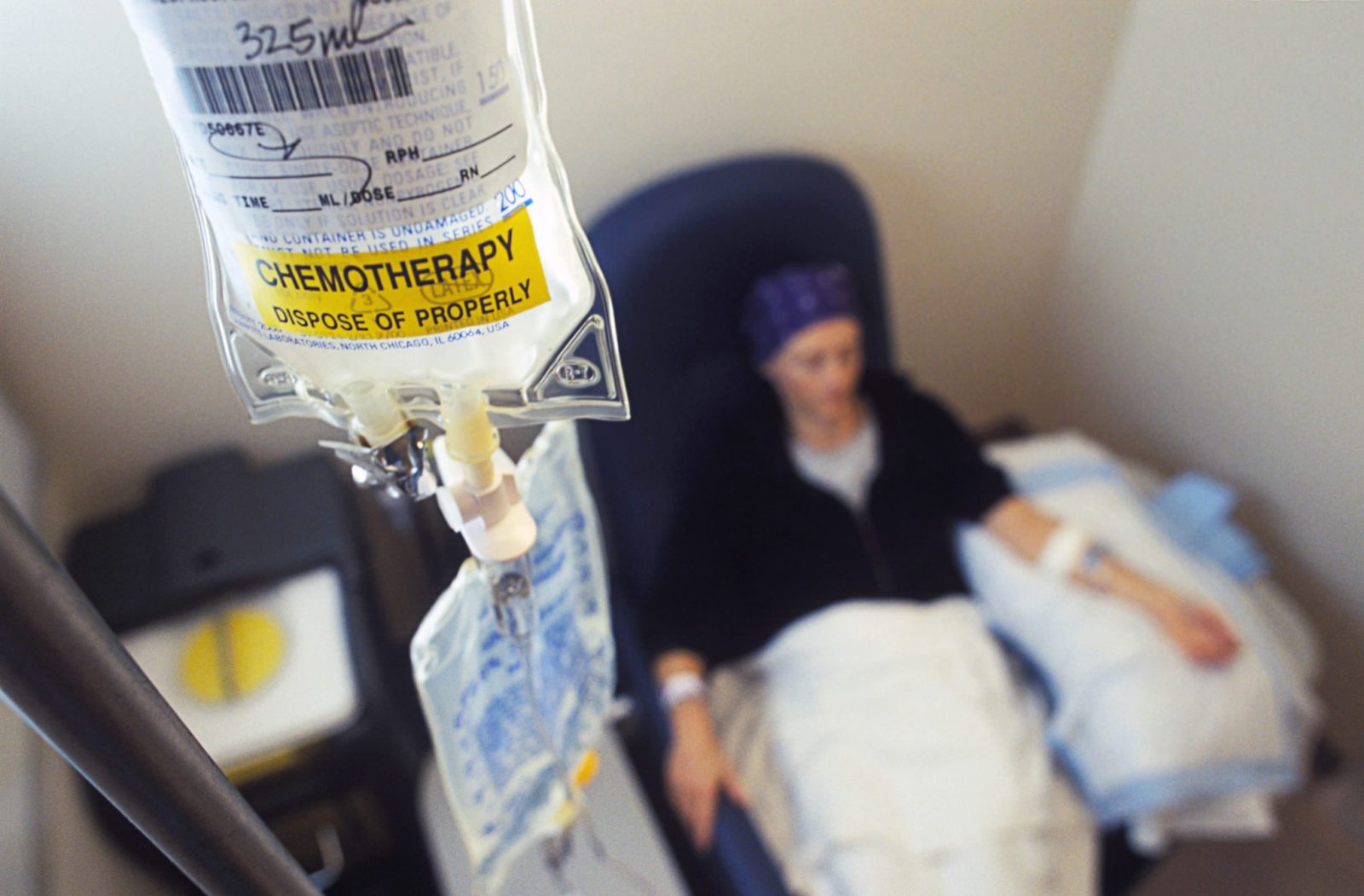
The FDA’s accelerated drug approval program is failing to protect cancer patients
Drug approval is a process that should be and, for the most part, is rooted in rigorous science. However, there is always a countervailing pressure to approve new drugs rapidly, particularly in cancer. That's why the FDA created the accelerated approval program in the early 1990s. Unfortunately, increasingly this approval process appears to be failing us in oncology. Reform is needed.

