A study published in 2001 reported the finding that antioxidants and zinc supplements slow the progression of age-related macular degeneration (AMD). Based on this study, eyecare providers routinely recommend these supplements to patients with AMD. Since then we have learned a great deal about the genetics of macular degeneration. Genes play a major role in the risk of developing AMD and in the progression of AMD. Numerous genes have been identified.
A group of investigator has published research promoting the assertion that the standard combination of antioxidants and zinc may result in sub-optimal outcomes for AMD patients with specific genetic fingerprints. If their assertions are true, genetic testing would be necessary to ensure that patients are receiving the optimal combination of supplements. A second group of investigators has performed their own analyses, criticized the methods of the first group, and vociferously challenged their recommendations. This debate has become quite contentious, and played out in numerous articles, counter articles, and letters to editors.
This debate has tremendous public health implications. The prevalence of AMD is so high that even small incremental changes in outcome affects the lives of millions of patients. It is also in interesting case study in critical analysis of conflicting opinions. In this post I will drill into the arguments on both sides and try to find the best answer based on the available data.
Background: What is age-related macular degeneration?
Courtesy of the National Eye Institute, National Institutes of Health
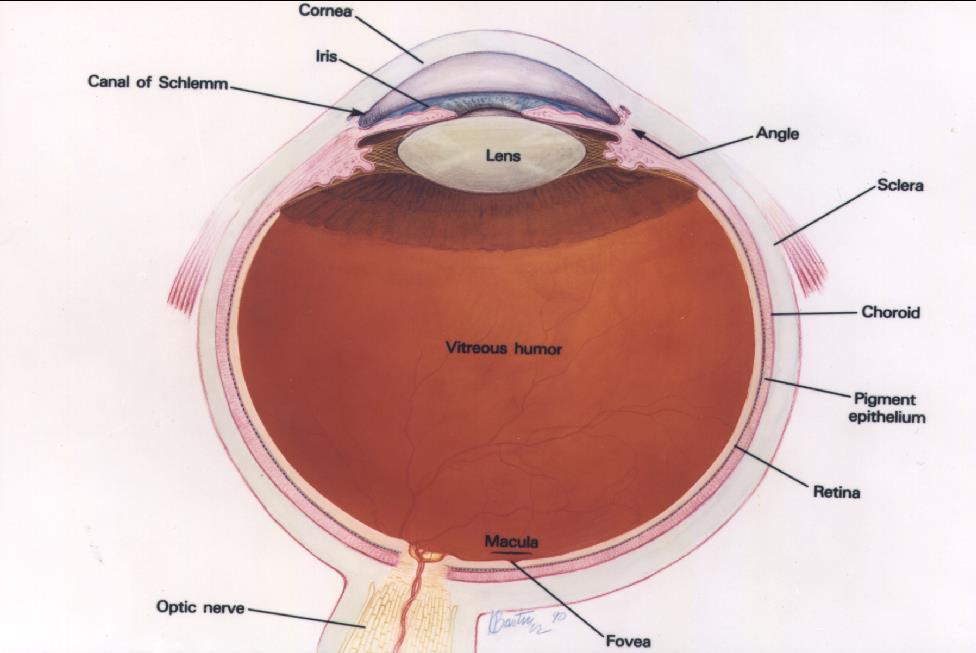
The retina lines the inner wall of the eye like wallpaper, and functions like the film in a camera. The cornea and lens, in the front of the eye, focus light on the retina. The retina send information to the brain via the optic nerve. The brain interprets the signals from the retina as vision. The macula is the anatomic and physiologic center of the retina. It is, quite literally, the center of your visual world. It is responsible for the sharpest, most detailed vision and needs to be healthy for reading and other detailed visual tasks.
ARMD occurs in two forms, or stages, referred to as “dry” and “wet”. Most patients have the dry type. In the early stages, patients may have no symptoms. Over time, there is often slow anatomic and functional deterioration of the macula. Patients may experience a variety of symptoms including, blurriness, loss of contrast sensitivity, distortion, and blind spots in or near the center of their vision, but the progression tends to be quite gradual, over many years.
Wet (or exudative) disease is the most feared form of ARMD. Only a minority of patients develop wet ARMD, but those that do often experience a rapid, catastrophic loss of vision over a period of weeks or months.
Chances are you know someone with AMD. If so, it is likely that an eye-care professional has recommended they take nutritional supplements to slow the progression of the condition. If you are a regular reader of SBM, you know that aside from overt nutritional deficiencies, vitamin supplements have generally exhibited no health benefits. AMD may be an exception.
AMD is a serious public health concern, with a substantial global burden. The prevalence is estimated to be 8.69% among older adults; substantially higher among those of European descent. By the year 2020 it is estimated that globally nearly 200 million individuals will have macular degeneration, increasing to 288 million by 2040.

Simulated vision for a patients with macular degeneration. Image courtesy of the National Eye Institute, National Institutes of Health
Recent advances have led to effective pharmacotherapy for exudative AMD. Although highly effective, this form of treatment is expensive, inconvenient, and uncomfortable for the patient. Patients with AMD live in fear of loss of vision and resulting loss of independence. Sadly, the fears become reality for many. The fear of vision loss prompts some patients to seek dubious and dangerous treatments. The American Academy of Ophthalmology has more information on AMD.
Nutritional supplements and macular degeneration: the AREDS Studies
In 1991, the Age-Related Eye Disorders Study Group (AREDS) reported a randomized, placebo-controlled clinical trial demonstrating that patients with age-related macular degeneration derived benefit from taking a combination of antioxidants and zinc. Compared to placebo controls, patients taking the antioxidants and zinc were less likely to progress to advanced stages of AMD. A second study, AREDS 2, published in 2013 resulted in some modification of composition of the antioxidants recommended for patients with macular degeneration. These two studies have become canon in the ophthalmologic literature, and inform clinicians’ recommendation for their patients with macular degeneration.
Genetics and macular degeneration
Macular degeneration is a disease influenced by a complex interaction of genetic and environmental factors. Twenty or more genes have been identified that influence the incidence and progression of macular degeneration, but two seem to be the most influential: Complement Factor H (CFH) and the Age-Related Maculopathy Susceptibility 2 Gene (ARMS2). The expression of these genes interacts with each other and with behavior/environmental factors such as age, smoking, diet, and BMI.
Everyone receives one copy (or allele in gene-speak) of the CFH gene from their mother and one from their father. Similarly they receive one allele each of the AMRS2 gene from their mother and father. Each allele may be the normal variant or may be a variant conferring additional risk of AMD. Each individual carries 0, 1, or 2 “risk alleles” for the CFH gene and 0, 1, or 2 risk alleles for ARMS2.
Pharmacogenomics and macular degeneration
Pharmacogenomics is the study of how genes influence an individual’s response to drugs. Pharmacogenomics is part of a movement branded as personalized medicine, or precision medicine, with the promise of being able to customized therapies based on the unique characteristics of each individual patient. In some fields of medicine, such as oncology, pharmacogenetics is increasingly important is making therapeutic decision. Based on the known genetic influences on macular degeneration, it is entirely plausible that a patient’s genetic profile might influence their response to treatment, including nutritional supplements. A group of researchers set out to test this hypothesis, and published a provocative and potential very disruptive study (Awh et al 2013).
The debate begins: Genotype influences response to AREDS supplements
The AREDS study was a publicly (National Institutes of Health) funded study. A subset of patients for the study had blood drawn and banked for future research. The study data, and the material from the genetic bank, were made available to independent researchers. Awh and collaborators obtained specimens and performed genetic analysis on a subset of 989 patients from the original AREDS study.
Awh’s analysis suggested that various combinations of CHF and ARMS2 risk factors influence response to supplements, to that extent that patients with certain genotypes may be harmed by the nutritional supplements recommended based on the AREDS studies. The authors of the paper recommended individual genetic testing of patients with macular degeneration to inform supplement recommendations.
The debate continues: Genotype does NOT influence response to AREDS supplements
The leadership of the AREDS study was quick to respond. A response article by Chew et al. attempted to discredit the Awh et al. study and presented a contrary analysis. Since the publication of that response, there has been a fierce controversy, played out in the literature, with articles, counter-articles, letters to the editor, and various editorials.
Yes it does!
Awh et al. published another article providing additional analysis on their first article. One of the criticisms of their first paper was lack or statistical significance for differing responses among the 9 genotypes. This time they sorted the 9 genotypes into 4 categories, and now were able to show statistically significant outcomes that differed from the original AREDS study results. They doubled down on their recommendation of individual patient genotyping to guide nutritional supplement advice.
Does not!
Chew et al. replied with a new analysis. They performed genetic analysis on an additional 526 patients from the AREDS study. The recreated the categories used by Awh et al., and found that this new group did not behave at all like Awh’s larger group. The group analyzed by Chew et al did not replicate the results of Awh, et al. Chew’s patients looked much more like the overall AREDS study population.
And so on…
The two camps continued to argue their interpretations of the data in letters and response letters to the editor and at high profile meetings.
Agree to disagree? Let’s look at the evidence
Here we have 2 groups. Each group has done its own analyses and reviewed the analyses of the other. Based on exactly the same information they have come to opposing conclusions. The Awh group feels they have convincingly demonstrated that genotype is necessary to make optimal supplement choices for patients with AMD. Chew and colleagues assert that the data are not sufficient to override the recommendations of the AREDS studies. How could this have happened? How could two groups of scientists reach such disparate conclusions based on the same data? Who is right?
Conflicts of interest
Conflicts of interest are common in research, and do not necessarily connote any impropriety in the conduct or interpretation of a study. Journals generally have strict rules about the disclosure of potential conflicts of interest for papers they publish. It is worth noting these disclosures when critically reviewing research. The first paper by Awh et al. has 5 authors. Awh, the first author discloses he is a consultant, member of the Scientific Advisory Board, equity owner, and patent holder for a company called ArcticDx. Another author is listed as consultant to ArcticDx, another is employee, equity owner, and holds patents for ArcticDx, and another is consultant and member of the Scientific Advisory Board for ArcticDx.
ArcticDx is introduced thusly on its website:
Welcome to ArcticDx Inc., a molecular diagnostic company with a focus on commercializing genetic discoveries in human healthcare. The company has a High-Complexity Genetic Testing Laboratory in Grand Rapids, Michigan. Arctic Medical Laboratories is CLIA and CAP certified and provides laboratory services to doctors nationally and internationally.
Among the genetic tests offered by ArcticDx are two relevant to AMD. MaculaRisk is a genetic test aimed at predicting the risk of progression for patients with AMD. And then there is VitaRisk:
Vita Risk is a pharmacogenetic test designed to aid in the selection of appropriate eye supplement formulations for a patient diagnosed with intermediate Dry Age-related Macular Degeneration.
The conflict of interest is clear. Part of ArticDx’s business depends on selling a genetic test. The rationale for the test is the assertion that different genotypes need different permutations of nutritional supplements.
The counter-analysis written by Chew et al had nine authors. Three of the authors reported patent royalties (of uncertain relevance) via the university for whom they work. One of these three also disclosed that they had provided consultancy services to a pharmaceutical company (with no obvious relevance to the issues under consideration). Another author reported patent royalties for AMD testing through a different university. The study was supported by the National Institutes of Health (NIH). It was disclosed that:
The NIH holds a royalty-bearing license issued to Bausch & Lomb for the Age-Related Eye Disease Study Supplement.
In other words, the NIH collects royalties from the company that markets and sells supplements recommended by the AREDS studies. If fewer patients receive the AREDS-recommended supplement combination, the NIH could lose revenue generated by their royalty agreement with Bausch and Lomb. None of the authors would profit directly from this arrangement, but two of the authors, including the first author (Dr. Chew), are employees of the National Eye Institute of the NIH.
Dr Ferris reported holding a patent for the Age-Related Eye Disease Study (AREDS) formulation with Bausch & Lomb.
Although not an author in any of Chew’s studies referenced so far, Frederick Ferris III, MD is a high-ranking official at the National Eye Institute, and Dr. Chew’s supervisor. He was integrally involved in the AREDS studies and has personally received royalties from Bausch & Lomb. His conflict of interest is disclosed on relevant AREDS publications on which he is an author.
A summary of the various financial conflicts is summarized in this article, which also hints at the animosity between the individuals on the two sides of the debate.
Conflicts of interest may raise red flags, but in the end, research findings stand or fall based on their own merits. In the next section I will take a deeper dive into the research methods for the various studies relevant to this debate.
More about AREDS
The strongest evidence of benefit of nutritional supplements for AMD came from the Age-Related Eye Disorders Studies (AREDS). The results of AREDS were surprising but generally persuasive. The first AREDS study was a multi-center randomized, placebo-controlled clinical trial. There were four treatment groups. Group 1 received antioxidants (Vitamin C, Vitamin E, and ß-carotene). Group 2 received zinc. Group 3 received antioxidants and zinc. Group 4 received neither antioxidants nor zinc. Each group received placebo versions of the supplements they were not assigned.
At baseline, patient fundus photos were graded and assigned a category of macular degeneration (a measure of severity). The endpoint was development of “advanced AMD.” For patients entering with category 3 or worse macular degeneration, the group assigned to antioxidants and the group assigned to zinc both had results numerically better than placebo. The group receiving antioxidants AND zinc did the best, surpassing the placebo group by a statistically significant margin.
In the chart below, each line represents the proportion of patients in a treatment group who progressed to a more advanced stage of macular degeneration at consecutive points in time. A shallower slope in the line means that patients in that group progressed at a slower rate, and therefore represents a more favorable outcome. Note that patients in the antioxidants plus zinc group had slowest rate of progression to advanced AND, therefore the best outcome among the treatment groups.

AREDS Report No. 8, Arch Ophthalmol. 2001;119(10):1417-1436.
The AREDS 2 study had a very complicated design, but when the dust settled, the only change to the AREDS recommendation was the substitution of lutein and zeaxanthin for ß-carotene. This was largely due to a known increase in the risk of lung cancer among smokers and former smokers taking ß-carotene.
The AREDS studies are not without their critics (possibly a subject for another SBM post), but the results have been widely accepted among eyecare professionals. Recommendation of AREDS-based nutritional supplement of antioxidants plus zinc has become standard of care for patients with AMD.
The case for genetic testing
Awh round 1
In their first paper, Awh and colleagues performed a genetic analysis on a subgroup of 989 patients from the first AREDS study and segregated them by genotype for CHF and ARMS2. This produced nine distinct genotypes. The analyzed a variety of interactions between genotype, AMD progression, and response to dietary supplements. The main conclusions of the paper arise from the figure and table below. The patients are sorted into groups based on the number of risk alleles for CHF (columns) and ARMS2 (rows).

From: Awh et al. CFH and ARMS2 genetic polymorphisms predict response to antioxidants and zinc in patients with age-related macular degeneration. Ophthalmology. 2013; 120(11):2317-2323. doi:10.1016/j.ophtha.2013.07.039.

From: Awh et al. CFH and ARMS2 genetic polymorphisms predict response to antioxidants and zinc in patients with age-related macular degeneration. Ophthalmology. 2013;120(11):2317-2323. doi:10.1016/j.ophtha.2013.07.039.
Based on these data the authors concluded:
In this analysis, patients with no CFH risk alleles and with 1 or 2 ARMS2 risk alleles derived maximum benefit from zinc-only supplementation. Patients with one or two CFH risk alleles and no ARMS2 risk alleles derived maximum benefit from antioxidant-only supplementation; treatment with zinc was associated with increased progression to advanced AMD. These recommendations could lead to improved outcomes through genotype-directed therapy.
Criticisms of study
Awh and coauthors did an unplanned subgroup analysis on a non-random sample of patients in the AREDS study. They further subdivided this sample into smaller groups based on genotype. These reasons limit the authority of these data for making therapeutic recommendations. It can be useful to perform analyses like this as a means of generating hypotheses to be confirmed or rejected based on additional research.
By dividing 989 patients into 9 genotype groups, they create small sample sizes. The genotype groups had as few as 10 patients. 5 of the 9 groups had fewer than 70 patients. The patients in each of these groups had been randomized among four treatment groups, creating even smaller samples. When slicing and dicing patients into this many small groups, one is almost certain to find that some groups deviate from the pattern of the original population. These variations could be due to true differences, but could be due to chance alone. Note that in the table above they list the “best Age-Related Eye Disease Study Treatment.” The “best treatment” conclusions are based on observation of trends but no statistical justification is reported. The numerous subgroups and lack of any statistical analysis render these data uninterpretable for making clinical decisions.
Awh round 2
In response to criticisms of the first paper, they published a second. This one introduced some additional analyses of the same data. They sorted the 9 genotypes into 4 groups. I have taken the liberty of annotating the figure from the first paper to show how they have sorted the genotype groups for the second paper. The authors describe these as “natural genotype groupings.”

Adapted from: Awh et al. CFH and ARMS2 genetic polymorphisms predict response to antioxidants and zinc in patients with age-related macular degeneration. Ophthalmology. 2013;120(11):2317-2323. doi:10.1016/j.ophtha.2013.07.039.
Analyzing for outcomes for each of the 4 genotype groups,

From: Awh CC, et al. Ophthalmology. 2015;122(1):162-169. doi:10.1016/j.ophtha.2014.07.049.

From: Awh CC, et al. Ophthalmology. 2015;122(1):162-169. doi:10.1016/j.ophtha.2014.07.049.
The authors conclude:
The benefit of theAREDS formulation seems the result of a favorable response by patients in only 1 genotype group, balanced by neutral or unfavorable responses in 3 genotype groups.
Based this conclusion they doubled down on the recommendation of customizing nutritional supplements based on individual patient genotype for ARMS2 and CFH.
Criticisms of the study
This remains an unplanned analysis of a nonrandom sample of patients from a larger clinical trial. This sample was further subdivided into genotypes. All these factors justify caution in generalizing these data. However, by sorting nine genotypes into four categories, they have generated large enough sample sizes to squeeze out some results that appear to be statistically significant. Are these analyses valid? Are the conclusions justified?
Chew and co-authors clearly do not think so. They published an analysis of a new data set and critique of the Awh study. They performed genetic analysis on a sample of 526 unique patients from the original AREDS study (referred to as the “residual cohort” in the paper and in the figure below). Although their sample size was smaller, they argued that if Awh’s findings were real, an independent sample from the same study population should exhibit similar trends, and if they did, it would serve as validation of Ahw’s analysis. In fact, when grouped and analyzed in the same way, Chew’s outcomes were much different than Awh’s, and were much more reminiscent of the results of the original AREDS study. This is clearly displayed in the following figure.
In the figure below, bars rising above the horizontal line indicate that the treatment group did better than placebo, whereas bars below the horizontal line indicate the group did worse than placebo. The height of each bar reflects the magnitude of the difference from the placebo group. The analysis on the left side of each box depicts Awh’s data and the analysis on the right side of each box depicts Chew’s analysis.

From: Chew et al, Ophthalmology. 2015;122(1):212-215. doi:10.1016/j.ophtha.2014.10.012.
Note that in genotype group 1, Awh’s analysis showed that the patients receiving antioxidants had the best outcomes (in contrast to the AREDS 1 study). In Chew’s analysis (residual cohort), patients randomized to antioxidants plus zinc had the best outcomes, just as they did in the AREDS study. In Genotype group 2, Awh showed that patients receiving any supplements did worse than placebo. This could be a serious concern, but Chew showed that patients receiving antioxidants, or antioxidants plus zinc did better than placebo, as in the original AREDS study. In Chew’s analysis, contrary to Awh, no genotype group was disadvantaged by being randomized to what became the AREDS-recommended formula of antioxidants plus zinc. Chew and coauthors proclaimed:
One should not deprive patients of a therapy that has been proven to have significant public health impact on the basis of a statistically flawed, not replicated retrospective analysis of existing data.
Who is right?…Experts weigh in
I have looked the data and the arguments of both sides, and I agree with Chew and coauthors. Awh’s findings are provocative but not persuasive. Various experts and professional organizations have communicated the same sentiment via editorials and letters to the editor. This includes a leading biostatistician and epidemiologist, a prominent ophthalmic geneticist, and a genetics task-force from the largest organization of American retina specialists. Clearly, the consensus of expert opinion does not accept Awh’s recommendation for genotyping of patients to inform selection of supplements for macular degeneration.
But Awh’s results were statistically significant!
In Awh’s first paper he and coauthors took a non-random sample from the study and further subdivided into nine genotypes. The data looked provocative, but not backed up with statistical analysis. In Awh’s second paper, he sorted the nine genotypes into four categories (genotype groups), and was able to generate some statistically significant analyses to reinforce the conclusions from their first paper. This is problematic for a couple of reasons. First of all, this remains an unplanned retrospective analysis of a non-random subgroup from a much larger study. This raises many cautionary flags. In the next step they reassembled the nine genotypes into larger “genotype groups.” It is important to note that the composition of these groups was defined after they had seen the data for each genotype. This is an enormous red flag. Why is this important? Imagine you have 20 cards to sort into four poker hands. You have the flexibility to sort any card into any hand. If the cards are face down, every hand would have an equal probability of being the winner and you would not know the outcome of the game until you turned the cards over. If you begin with the cards face-up and you were so-motivated, you could control the position of the winning hand every time.
Look again at this figure showing the way Awh et al. sorted genotype groups for their analysis.

Adapted from: Awh et al. Ophthalmology. 2013;120(11):2317-2323. doi:10.1016/j.ophtha.2013.07.039.
The sorted nine groups into four categories. Category 2 contains only one genotype group. Categories 1 and 4 have two genotype groups each. Category 3 has four genotype groups. There are hundreds of ways nine items can be sorted into a distribution of 1:2:2:4. But it is more complicated than that. They could have sorted into categories of 1:2:3:3, or 2:2:2:3, etc, each with hundreds of permutations. Why not three categories…3:3:3, 2:3:4? There are literally thousands of ways they could have sorted the nine genotypes into genotype groups. If the investigators had a pre-specified hypothesis that sorting the genotypes in one particular way would yield a particular outcome, then confirmed that outcome with data, their conclusions would have much more credibility.
Even with random data, if you split the data into small groups then, with full knowledge of the data, creatively sort these into larger categories, one can often (if so motivated) “find” a pattern. This is somewhat analogous to gerrymandering in the drawing of voting districts. In a rare (and refreshing) display of academic snark, Chew et al. demonstrated this point. They “proved” that patients born under the signs Cancer or Aries had negative outcomes in the Awh cohort, but good outcomes in the Chew cohort.
However, despite the methodological weaknesses and the expert consensus against Awh’s recommendations, some have been persuaded by them.
A fresh look at stale data!
Awh et al. and Chew et al. voluntarily submitted their data to be distributed to three prestigious groups of academic statisticians (the identities of the statistics teams were disclosed in the publication, but not to Awh et al. or Chew et al. at the time of analysis). The teams worked independently and used different methods to explore the data from the two groups. After performing their analyses, the groups collaborated to write a report (currently “in press” but available online). Remember these statisticians were not just critiquing the analyses of the two feuding groups, they were performing independent analysis of the actual data provided to them by the two groups of investigators. The results could hardly be less ambiguous and is thusly summarized in the abstract of the paper:
We found errors in the data used to support the initial claim of genotype–treatment interaction. Although we found evidence that high-risk patients had more to gain from treatment, we were unable to replicate any genotype–treatment interactions after adjusting for multiple testing. We tested 1 genotype claim on an independent set of data, with negative results. Even if we assumed that interactions in fact did exist, we did not find evidence to support the claim that supplementation leads to a large increase in the risk of advanced AMD in some genotype subgroups.
They concluded:
Patients who meet criteria for supplements to prevent AMD progression should be offered zinc and antioxidants without consideration of genotype.
Case closed!(for now)
The evidence for pharmacogenomic selection of supplements for patients with AMD failed to persuade experts in the field and more importantly failed statistical validation by independent expert statistical teams. In this author’s opinion this debate should be considered settled, unless more persuasive data emerges.

