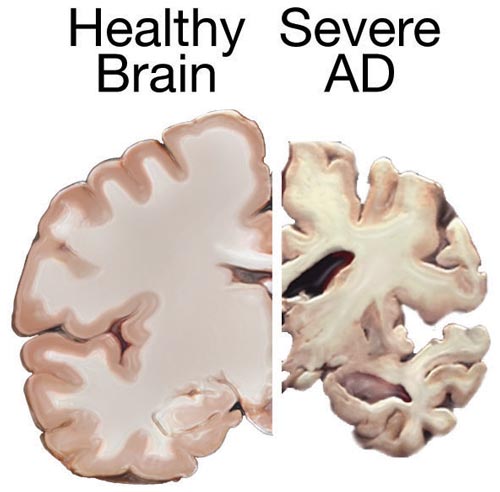
Post-mortem cross sections of a healthy brain (left) and a brain with advanced Alzheimer disease (right), showing characteristic shrinkage.
SBM essentially advocates for an ironic-sounding holistic approach to scientific evidence. All evidence should be considered in its proper context with an eye toward the strengths and weaknesses of each kind of evidence, and in the context of the institutions of science and medicine. SBM represents a higher standard of overall evidence, which we feel is justified given the degree to which medical interventions are adopted prematurely (as evidenced by later reversals).
At the same time there are those, in the minority but with an established presence, who are essentially arguing for lowering the standard of science in health care. They exist on a spectrum, at one end including those who would abandon science entirely in favor of spirituality and philosophy-based medicine. At the other end are those who claim to endorse science but want to change the rules of scientific medicine to include a much lower standard of evidence. This is more pseudoscience than antiscience. Chief among them, in my opinion, are proponents of what they call “functional medicine.” Functional medicine essentially uses science incorrectly, but still cloaks itself with the imprimatur of science.
The MEND Protocol
As an example of how functional medicine uses scientific evidence incorrectly, we have the MEND Protocol for Alzheimer’s disease (AD).
AD is a complex neurodegenerative disease that results in the death and dysfunction of neurons in the brain, causing a slow global cognitive decline over years. This is one of those diseases where we have a great deal of research and knowledge about what is happening in the disease, but we have not been able to put it all together and determine what the ultimate cause is.
The problem with neurodegenerative diseases in general is that a series of complex changes occur in the cells and we don’t know what is cause and what is effect. A lot of things happen when neurons die, but are those things causing the neurons to die or just a result of the dying process?
In AD, for example, we see the buildup of certain proteins, causing plaques and neurofibrillary tangles. We also see that some proteins have changed their conformation, similar to what happens in Creutzfeldt-Jakob disease (the human version of bovine spongiform encephalopathy or mad cow disease). Perhaps oxidative stress is playing a role, or neurotoxic neurotransmitters. Some gene variants present a higher risk of developing AD. There is impaired axonal transport, and there is inflammation.
Inflammation is always tricky because it is virtually ubiquitous when cells are dying, and is also part of the healing process. The immune system is the clean-up crew, which reacts to damaged and unhealthy cells. So when we see markers of inflammation we don’t know if the inflammation is causing the damage or a reaction to it, or both. The inflammation may also be reactive, but then accelerate the damage.
As a result of this complexity we have lots of clues but few clear answers. There have been many clinical trials which essentially fix one of the markers for AD, but they so far have not altered the course of the disease.
In general we need high quality clinical trials, with proper blinding, to know if a specific intervention actually works, regardless of how plausible it seems based on the basic science. When dealing with a disease as biologically complex as AD, it is especially necessary because the number of false leads is massive.
The Functional Medicine approach
The MEND Protocol is a functional medicine approach to AD. According to the website of Muses labs, which owns the rights to the protocol (as evidenced by the “TM” after name – always a red flag):
MEND Protocol is designed to address the active underlying pathways for Alzheimer’s disease including metabolic issues, toxicity, inflammation, and mitochondrial damage. The therapeutics recommended by the MEND™ Protocol offer a way to bring these mechanisms into a state of enhancing a participant’s cognitive health – and maintaining that control over time. These factors and their complex interrelationships must be personalized based on an individual’s specific needs.
Due to the complexity of the personalization process, the Protocol is created via health management software. Our algorithms are able to process a wide range of data on an individual’s health status and recommend specific interventions matched to each individual.
Essentially their approach is to address all the markers in AD, all the things that basic science studies show might be off, without addressing the issue of whether or not these markers actually cause or drive AD. They then add the further layer of complexity of “personalized” medicine.
Personalized medicine is similar to functional medicine, except that it is reasonable in theory. If we can learn how to predict (with genetics, mostly) how an individual patient will respond to a treatment, we will be able to have a better outcome then just applying population-based data.
The challenge is, we need a lot of data about how individual factors affect the response to interventions. Many people promising personalized medicine currently, in my opinion, are skipping this important step. It becomes pseudoscience because it is massively premature, trading on the hype and promise of the personalized approach, but skipping over all the tedious science.
The result of their function and personalize approach is a salad of technobabble:
The analysis algorithm recommends both pharmacological and non-pharmacological components. For example, if synaptic reconstruction and maintenance is needed, then multiple biological mechanisms may require normalization, enhancement, or administration. Examples of these underlying biological mechanisms include: periodically activating autophagy, blocking prionic tau amplification, increasing beta-amyloid clearance, inhibiting beta-amyloid oligomerization, minimizing inflammation, normalizing neurotrophic factors, reducing ApoE Ɛ4- mediated signals, reducing stress, reducing tau phosphorylation, restoring cholinergic neuro- transmission, and reversing memory loss. Assessing the status of these biological mechanisms involves quantifying and observing hormonal balance, citicoline, C-reactive protein and other inflammation-related markers, diet, exercise, homocysteine, omega-3 acids, sleep, and so on. Interventions targeting specific biological mechanisms are then prioritized and prescribed to optimize key biological mechanisms. Medication doses are specified to an individual’s needs. Individuals are re-tested periodically and the protocol is updated as necessary.
How, exactly, do they know if a patient requires “synaptic reconstruction” and what evidence do they have that there is an effective treatment? It sounds very sciencey and impressive, and that is apparently the idea, but they are getting at least several steps ahead of the science.
A recent study
A study looking at the clinical effects of the MEND protocol was just published: “Reversal of cognitive decline in Alzheimer’s disease.” Despite the suggestive title, the methods of the study are preliminary.
The therapeutic approach used was programmatic and personalized rather than monotherapeutic and invariant, and was dubbed metabolic enhancement for neurodegeneration (MEND).
Essentially this is an observational study, a case series of 10 patients. This is reasonable as a preliminary study design, but it should not be used to support claims of efficacy – it is not an efficacy trial. All 10 patients improved in the trial.
The data, of course, is unblinded, meaning that it is very unreliable. In my experience as a neurologist, cognitive function can be very subjective. It is affected by mood, by transient things such as sleep, and there is also a huge element of subjective perception.
Some of the subjects did better on neuropsychological testing, but there is a known practice effect to such standardized tests. Patients always do better on the second taking.
They present 10 cases, but there is no mention of how these 10 cases were selected and if they are representative.
I also note that the authors refer to patients as having “Alzheimer’s disease” when in fact they should have referred to them as “Alzheimer’s type dementia.” AD is a pathological diagnosis, and there is no mention of brain biopsy on any of the cases. They met clinical criteria for Alzheimer’s type dementia, which is not the same as confirming AD.
Therefore, it is quite possible that some of these patients had a self-limiting process in the first place. This is exactly why randomization and blinding are necessary.
Conclusion: Dubious science, based on questionable theory
The approach of the MEND protocol is highly dubious in that it extrapolates wildly from basic science to clinical application, in a disease known for its horrific complexity. They further add another layer so complexity by claiming to “personalize” the protocol.
While the current case series is certainly provocative, I do not think it is reasonable to use this level of evidence to make clinical claims. This is especially true given the extraordinary nature of those claims.
What we need now is a randomized double-blind placebo controlled trial. They can still use their personalization – just do the protocol on every subject, while giving half actual treatment and the others placebos.

