Author’s note: The FDA has asked for public comments on the regulation of homeopathic products. The Society for Science-Based Medicine’s Comment follows, modified for this format. The Comment is based in part on two previous posts, “How should the FDA regulate homeopathic remedies?” and “Homeopathic industry and its acolytes make poor showing before the FDA.” The comment period closes August 21, 2015.
Society for Science-Based Medicine
Comment: Homeopathic Product Regulation: Evaluating the Food and Drug Administration’s Regulatory Framework After a Quarter-Century
All homeopathic products on the U.S. market today, whether over-the-counter (OTC) or prescription, fall within the definition of “drug” in the Food, Drug & Cosmetic Act of 1938. The overwhelming scientific consensus is that homeopathy is highly implausible, unsupported by scientific evidence, ineffective in treating illness and, when relied upon instead of actual medicine, dangerous and even deadly. Yet the FDA has, without statutory authority, exempted homeopathic drugs from the regulatory scheme mandated by federal law. In accordance with its consumer protection mandate, the FDA should take immediate action to remedy this by requiring that all homeopathic drugs comply with the same statutes and regulations as all other OTC and prescription drugs.
Introduction
The Society for Science-Based Medicine welcomes the FDA’s examination of homeopathic prescription and OTC drug regulation. In many respects, the current regulation of homeopathic drugs resembles that of all drugs prior to the passage of the Food, Drug & Cosmetic Act in 1938 (FD&C Act). At that time, there was no premarket regulation. The FDA was limited to post-market enforcement actions against adulterated or misbranded drugs. Drug manufacturers controlled the premarket process, much as the homeopathic industry controls the premarket process today. This includes the determination of which homeopathic ingredients are included in the Homeopathic Pharmacopœia of the United States (HPUS) by a private, non-governmental organization, the Homeopathic Pharmacopœia Convention of the United States (HPCUS), as well as homeopathic manufacturer determination of indications for product use. Then, as now, the FDA promulgated labeling requirements, but there was no FDA process ensuring that the products were safe and effective for their intended use prior to their being marketed.
According to the FDA, Americans now spend about $3 billion a year on homeopathic drugs, which contain ingredients derived from plants, healthy or diseased animals or humans, minerals and various chemicals. These drugs can cause, and have caused, side effects, some of them serious. Yet, there is no reliable scientific evidence that these drugs are efficacious for any use. As FDA historian John Swann, Ph.D., noted, prior to Congress giving the FDA authority to regulate drugs, products on the market “were, at a minimum, useless remedies that picked the pocket of the user, but they could also be downright harmful,” an observation that describes perfectly homeopathic products today.
Obviously, a system widely recognized as insufficient over 75 years ago cannot be tolerated in 2015. It was this very insufficiency that prompted Congress to pass the FD&C Act and subsequent amendments further strengthening the FDA’s hand, laws from which the homeopathic industry has been largely, and curiously, exempt. Indeed, it is entirely unclear on what statutory authority the FDA bases its exclusion of homeopathic drugs from the pre-market regulatory process applicable to all drugs under federal law. There is certainly no such exclusion in the statutes themselves.
The FDA asked for public input on specific issues regarding regulation of homeopathic drugs. The Society’s comments follow.
- What are consumer and health care provider attitudes towards human drug and biological products labeled as homeopathic?
The public has spoken with its pocketbook. Consumers are unlikely to purchase a remedy for themselves and their families unless they think the product is safe and effective. These consumers are apparently unaware of several facts:
- There has been no objective, third-party confirmation of safety and effectiveness by the FDA or otherwise.
- Claims of efficacy are in direct contradiction to basic principles of physics and chemistry and no reputable scientific authority supports the fantastical postulates upon which homeopathic remedies are reputed to “work.”
- Clinical trials confirm the obvious: homeopathic drugs do not work as claimed.
There could be no more compelling evidence that the current system is inadequate than the public comments posted online at regulations.gov. As of this writing, there are almost 8,000 comments, the majority of them consisting of positive anecdotes about homeopathic drug use. Considering the fact that there is no evidence of efficacy, it is clear the homeopathic industry has the public fooled.
We see, over and over, comments that homeopathic remedies are “safe and effective” or “proven safe and effective” when the consensus of the scientific community is that they are not and, in fact, cannot be effective, and we know that safety remains largely unproven. One physician testified at the FDA’s hearing that “the majority of patients . . . that I encounter have no real understanding of what homeopathy is and often confuse it with herbal medicines.”
Many commenters claim that homeopathic remedies are effective for a wide range of illnesses and conditions, not all of them self-limiting and some quite serious. Some use the word “cure;” one claimed homeopathic drugs “cured people of serious diseases.” Another claimed that a homeopathic drug brought a seven-year-old out of a coma. Yet another said homeopathy was effective for a “vast range of ailments and conditions.” A random selection of about 350 of these comments included claims that homeopathic remedies are effective for:
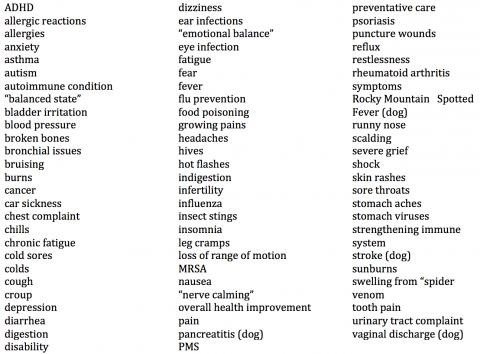
These consumers apparently have no understanding that the perceived “effectiveness” of homeopathic products could well be due to such things as the natural course of disease, motivated reasoning, placebo responses, regression to the mean, confirmation bias, conditioning, the post hoc ergo propter hoc fallacy, or the effect of other treatments. In fact, from a scientific standpoint, these are the only plausible explanations for the putative effectiveness of homeopathic products.
Misattributions of efficacy are exactly the sort of confounding factors that the premarket approval process is designed to weed out. By putting the onus on prescription and OTC drug manufacturers to show safety and efficacy, this process ensures that consumers will not be purchasing remedies that actually do them no good and allows calculation of the true benefit of the product itself so that a proper risk-benefit analysis can be performed. As it now stands, the homeopathic industry is allowed to exploit these other, plausible explanations for the perceived effectiveness of their products in order to sell unnecessary and ineffective remedies for their own financial gain.
If the FDA ever believed that use of homeopathic drugs by health care providers offered some measure of protection for the public, those beliefs were certainly belied by the comments of providers who employ homeopathic remedies in their practices. In fact, while the industry’s self-interest and the public’s lack of accurate information can explain the average consumer’s being misinformed, it is much harder to reconcile the use of unproven drugs rejected by the vast majority of medical practitioners, and for good reason, by those presumably educated in the scientific method.
Robert Dumont, MD, a pediatrician, testified that homeopathic products were “exceptionally versatile and efficacious for many medical problems,” that he has “prescribed homeopathic medicines in the hospital for premature infants,” and used it for “nausea, vomiting and losing weight” in a patient undergoing chemotherapy. Another MD, Karl Robinson, testified he used homeopathy for Hepatitis A. Younger Chug, MD, a pulmonologist, said he uses homeopathic products for asthma, chronic cough, croup and in situations where there is no effective drug, such as dyspnea due to vocal cord dysfunction. Another said homeopathy was “especially beneficial in our sickest patients.”
Amy Rothenberg, ND, testified she prescribed homeopathy for an autistic child. Another naturopath said that “time and again [homeopathy has been] very, very safe and effective as adjuvant therapy in restoring people’s health and vitality.” She said it had been “life changing, a child born, depression lifted, digestion improved.”
As the FDA is well aware, none of these uses are supported by adequate evidence of safety and efficacy.
- What data sources can be identified or shared with FDA so that the Agency can better assess the risks and benefits of drug and biological products labeled as homeopathic?
Before this question can be addressed, the FDA will have to determine what process it will use to evaluate these data sources, whatever they might be. As the FDA itself said in its notice of the public hearing, nothing in the FD&C Act exempts drugs labeled as homeopathic from the premarket approval process or the same safety and efficacy standards as all new drugs. There is no doubt that all homeopathic drugs meet the definition of “new drug,” because they are “not generally recognized, among experts qualified by scientific training and experience to evaluate the safety and effectiveness of drugs” for uses “prescribed, recommended, or suggested” by the label. Thus, homeopathic drugs must meet the same criteria as that applied to other new drugs, whether they are prescription or OTC.
As to the data sources themselves, the FDA is fortunate that the Australian National Health and Medical Research Council (NHMRC) recently completed a comprehensive evaluation of the evidence. The resulting analysis, “Effectiveness of Homeopathy for Clinical Conditions: Evaluation of the Evidence” (2015), concluded:
There is a paucity of good-quality studies of sufficient size that examine the effectiveness of homeopathy as a treatment for any clinical condition in humans. The available evidence is not compelling and fails to demonstrate that homeopathy is an effective treatment for any of the reported clinical conditions in humans.
This led to the NHMRC Statement on Homeopathy:
Based on the assessment of the evidence of effectiveness of homeopathy, NHMRC concludes that there is no health condition for which there is reliable evidence that homeopathy is effective.
As well, the UK House of Commons Science and Technology Committee, in its “Evidence Check 2: Homeopathy,” after a review of the evidence, concluded:
In our view, the systematic reviews and meta-analyses conclusively demonstrate that homeopathic products perform no better than placebos. We could find no support from independent experts for the idea that there is good evidence for the efficacy of homeopathy.
What cannot be tolerated, in our view, is lowering the FDA’s standards to permit the consideration of “data sources” widely rejected by experts qualified by scientific training and experience. The mechanism of action for homeopathy proposed by the homeopathic industry is inconsistent with basic principles of chemistry and physics. Indeed, as has been pointed out, what we understand now about chemistry and physics would have to be proven very wrong if homeopathy actually “works.”
As Tanya Kell, President of the North American Society of Homeopaths, admitted at the hearing, the “long-established record of safety and efficacy” claimed by the industry is largely “a compilation of anecdotal evidence.” It is unthinkable that any other FDA-regulated product would be allowed on the market based on anecdotes.
At this time, the FDA has defaulted to the HPCUS to determine what ingredients can be used in homeopathic drugs and to the homeopathic industry to decide, with no reliable evidence whatsoever, safety and appropriate indications for use. The industry’s conflict of interest is obvious. What may be less obvious is that the HPCUS is controlled solely by industry insiders. The HPCUS Board, which exercises final judgment regarding which ingredients are included in the HPUS, is composed of practicing homeopaths, homeopathic pharmacists, or current or former officials or owners of homeopathic products manufacturing companies.
Yet, the FDA has claimed, in testimony before Congress, that it applies the same evidentiary standards whether a therapy is labeled conventional or complementary and alternative. In 2000, an FDA official told a House Committee:
CAM is a broad term referring to treatments that are either unapproved or not widely accepted in this country. Treatments range from botanicals and animal extracts to biofeedback to visualization techniques, chiropractic, homeopathy, massage therapy, acupuncture, and prayer. As we have emphasized, FDA relies on evidence, and is required to do so under the FD&C Act, from adequate and well-controlled studies as its basis for approval, not on theories of healing, animal studies or strongly held beliefs. Complementary and alternative treatments are as readily studied in well-controlled trials as are conventional treatments . . . [Emphasis added.]
In sum, the FDA should use only data sources that meet the same requirements as those allowed for all other FDA-regulated drugs. Conversely, the FDA should reject scientifically implausible “theories,” anecdotal evidence, homeopathic “provings” and obviously biased industry sources as the standard for homeopathic drugs.
- Are the current enforcement policies under the CPG [Compliance Policy Guide] appropriate to protect and promote public health in light of the tremendous growth in the homeopathic drug market? Are there alternatives to the current enforcement policies of the CPG that would inform FDA’s regulatory oversight of drugs labeled as homeopathic? If so, please explain.
- Are there areas of the current CPG that could benefit from additional clarity? If so, please explain.
Given the fact that consumers who use homeopathic drugs are not aware of the overwhelming scientific consensus that homeopathy does not, and cannot, work, and that no robust clinical trial evidence for their efficacy exists, then it is quite obvious that the current CPG is inadequate. The fact that consumers do not confine their use to self-limiting conditions is further evidence of their confusion.
The only solution is to scrap the current CPG and apply the same regulations and policies to homeopathic drugs that the FDA applies to all other OTC drugs. (This same solution is applicable to prescription homeopathic drugs as well: regulate these drugs like all other prescription drugs.) If a homeopathic drug has never been properly evaluated to determine whether it is OTC or prescription, then that should be the first step. The rest of the regulatory process follows logically from there. As we have pointed out before, and as the FDA itself has said, there no statutory authority to regulate homeopathic drugs differently than all other drugs on the market.
If the FDA concludes that removing all homeopathic products from the shelves, because they’ve never been through the appropriate regulatory process, is impractical, interim steps could be taken until all homeopathic drugs can complete the process. As a first step, the FDA can, at the very least, apply the same product labeling rules to OTC homeopathic drugs it does to all other OTC drugs. This includes listing ingredients and ingredient amounts using the same terminology as is used for all other OTC drugs. In other words, the Latin names and reference to amounts in homeopathic dilutions should be abandoned. It would also mean that no homeopathic drug could claim to be safe and effective for any disease or condition until it met the same evidentiary standards as all other OTC drugs.
- Is there information regarding the regulation of homeopathic products in other countries that inform FDA’s thinking in this area?
The FDA has in place an extensive regulatory process designed to evaluate the safety and effectiveness of OTC and prescription drugs and to ensure that the public and health professionals have adequate information for their use. It is this system the FDA should employ for homeopathic drugs. To the extent other countries are employing any regulatory scheme that allows the sale of homeopathic drugs based on false claims of safety and effectiveness, that system simply suffers from the same deficiencies as those that plague the current system in this country. Consequently, they should not be adopted.
- A large majority of human drug products labeled as homeopathic are marketed as OTC drugs. These products are available for a wide variety of indications, and many of these indications have never been considered for OTC use under a formal regulatory process. What would be an appropriate regulatory process for evaluating such indications for OTC use?
There is no legal or practical reason why the FDA should continue to privilege homeopathy over all other drugs by indulging the homeopathic industry and ignoring basic science and the overwhelming evidence from clinical trials that homeopathy does not and cannot work. To date, the FDA’s rationale for doing so has been the presumed safety of homeopathic drugs, due to the fact that they contain anywhere from a small amount to no active ingredient. Safety aside, in light of the public’s, and health care providers’ obvious belief in the efficacy of homeopathic products for many conditions, some of them quite serious, the FDA can no longer afford to allow these misperceptions to go unremedied by regulatory action. Again, the appropriate regulatory process is the one already in place for other OTC drugs. This should include the payment of any fees required of other OTC drug manufacturers.
- Given the wide range of indications on drug products labeled as homeopathic and available OTC, what processes do companies currently use to evaluate whether such products, including their indications for use, are appropriate for marketing as an OTC drug?
We submit that the processes these companies use, whatever they are, are highly irregular to the extent they are based on homeopathic principles. There is a plethora of scientifically valid information evaluating homeopathic drugs. It is abundantly clear that the homeopathic industry has not availed itself of this information in evaluating “whether their products, including their indications for use, are appropriate for marketing as OTC drugs.”
To the extent the evaluation of homeopathic drugs by their manufacturers does not conform to the processes used by other OTC drug manufacturers, they must be scrapped. We do not believe there is a way to create some hybrid regulatory system supported by one foot resting in science and the other in pseudoscience.
- Do consumers and health care providers have adequate information to make informed decisions about drug products labeled as homeopathic? If not, what information, including, for example, information in labeling, would allow consumers and health care providers to be better informed about products labeled as homeopathic?
The answer to the first question is clear: Consumers and a handful of health care providers believe that homeopathic drugs are safe and effective for many uses and that the “theory” underlying homeopathic remedies is a tenable one. A necessary corollary to that conclusion is that they have inadequate information to make informed decisions about homeopathic drugs.
The fact that these products are labeled “homeopathic” (a fact mentioned by several homeopathy proponents at the hearing) begs the question. That means nothing if the consumer doesn’t understand what “homeopathic” means. Of course, to the homeopathic industry, it means that “like cures like” and that dilution combined with vigorous shaking makes the product more, rather than less, effective. Consumers testifying at the hearing and commenting online parroted this explanation, ironically demonstrating they have no idea what “homeopathic” really means: that the product has been produced by a method that virtually guarantees it will not work as advertised.
One speaker repeated the homeopathic industry’s latest attempt at reconciling their scientifically untenable “theory” with basic chemistry and physics: “we now know that water holds energetic information and the energy of each substance is stored within the homeopathic remedy.” Another said they are “effective through their energetic frequency and the effect they have on the cellular electrical energies.” Yet another, a naturopath, claimed that homeopathy can be explained by hormesis. All of these are nonsensical from a scientific standpoint.
This is exacerbated by the fact that ingredients are listed in Latin and dilutions are listed in a code used only by the homeopathic industry. In addition to these confusing labels, consumers commented that they relied on self-help books, the internet and personal recommendations, all unreliable sources of accurate information.
Proponents of homeopathic products testifying at the hearing gave examples of available online sources. However, these sources simply repeat the unproven claims that homeopathy is safe and effective for a wide range of diseases. For example, the Consumer Healthcare Products Association, a trade group, refers viewers to the websites of two homeopathic product manufacturers, Hyland’s and Boiron, hardly objective sources of reliable information.
The website of the National Center for Homeopathy, mentioned several times in testimony, contains information that can only be described as fantastical from a scientific standpoint. Likewise, the American Institute for Homeopathy website states that homeopathic remedies “stimulate the person’s own healing power,” which is nothing more than vitalism, a pre-scientific, and long-rejected, belief in an incorporeal “healing force” erroneously credited with healing powers. It falsely claims that homeopathic drugs are “deep-acting medicines [that] can be used to treat persons experiencing many kinds of medical conditions.”
Unfortunately, the FDA’s own website has almost no information about homeopathic drugs. This paucity is striking when compared to the plethora of information available for OTC and prescription drugs, as well as the many other products the FDA regulates, such as foods and medical devices. The National Center for Complementary and Integrative Health has limited information. Fortunately for those consumers consulting NCCIH, the material is refreshingly honest: the FDA does not evaluate homeopathic remedies for safety or effectiveness, there is little evidence that it is effective for anything, and although highly diluted some products have concentrations capable of causing adverse effects.
At the hearing, the homeopathic industry was unable to produce any evidence that the consumer understands the true nature of homeopathic remedies. One industry representative testified it might be helpful to require a disclaimer on the label similar to that on dietary supplements. However, this appears to have had little effect on the public’s misplaced belief that dietary supplements are proven safe and effective, despite the fact that the FDA does not evaluate supplements prior to market and the lack of evidence that supplements are indeed either safe or effective for their advertised uses. Of course, unlike dietary supplements, where there is specific statutory authority in the Dietary Supplement Health & Education Act (DSHEA) to use this disclaimer, nothing in the FD&C Act permits the FDA to avoid its regulatory mandate in this manner.
Of note, there is popular support for the increased regulation of dietary supplements. (Again, DSHEA, unlike the FD&C Act, does not require pre-market approval.) One survey reported in the medical literature found that a substantial majority of respondents, including regular dietary supplement users, were in favor of
- requiring the FDA to review the safety of new dietary supplements prior to their sale;
- providing increased authority to remove from sale those products shown to be unsafe; and
- increasing government regulation to ensure that advertising claims about dietary supplements are true.
Summary
The answer to the FDA’s questions is simple and can be summarized in a few words.
- Homeopathy is highly implausible, unsupported by scientific evidence, ineffective in treating illness and, when relied upon instead of actual medicine, dangerous and even deadly.
- The FDA should apply the FD&C Act as Congress intended. The law offers no exception for homeopathic products.
The Society for Science-Based Medicine is a 501(c)(3) tax-exempt charitable organization. Our mission includes advocacy on behalf of consumers to ensure that all government regulation affecting health care has a sound basis in science and the scientific method. For more information see www.sfsbm.org.
