
When it comes to drugs or dietary supplements, accuracy should be a given. What’s on the label should accurately describe what’s in the bottle. No exceptions. When it comes to ensuring the products we buy are of high quality, we’re all effectively reliant on regulation to protect us. As a pharmacist, I can’t personally verify that each tablet in your prescription contains the active ingredient on the label. I am dependent on a supply chain that may stretch around the world. While the product manufacturer may be reputable, it’s only a regulator that can realistically verify and enforce production to strict quality standards.
The same cannot be said for products like supplements and herbs which are regulated differently than drugs, and held to different, and in some cases, weaker standards. A weak regulatory framework, which doesn’t hold manufacturers to account, would be expected to result in a product of lower quality. And that’s exactly what you see in this most recent paper.
Two versions of galantamine
In this small but effective paper, Peter A. Cohen and colleagues set out to determine if label accuracy differs between prescription drugs and dietary supplements. They chose a chemical which is both. Galantamine, which can be naturally sourced from plants, is available in the United States as either a dietary supplement or as a prescription medication. As a drug, galantamine is approved by the FDA for the treatment of mild to moderate Alzheimer dementia. It is also sold as a dietary supplement marketed for cognitive conditions and “memory enhancement.” Other than some vitamins, I’m not sure what other products straddle both categories. So this was an ideal product to test.
In June 2023, all galantamine dietary supplements on Amazon.com labeled with both galantamine as an ingredient that also had Supplement Facts panel were purchased online. (The Supplement Facts panel was required to ensure the product was actually being marketed as a dietary supplement.) In September 2023, versions of all generic immediate-release prescription versions of galantamine were purchased.
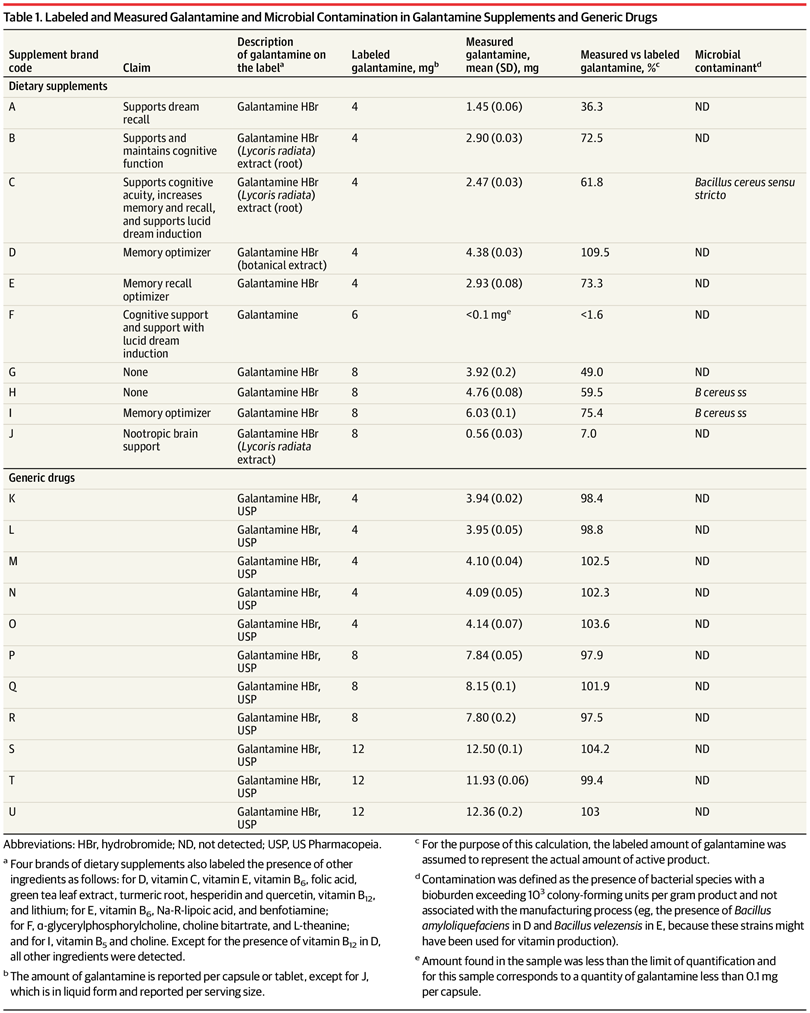
Products were then analyzed for the presence of galantamine, and the amount was quantified. Contamination with microorganisms was tested for as well.
The results
Drugs first:
Eleven brands of generic galantamine were purchased and tested, in various strengths. The actual content of galantamine in the generic drugs ranged from 97.5% to 104.2% of the labeled content. This is better than the United States Pharmacopeia standard, which specifies 90% to 110%. None of the products were contaminated with bacteria.
Supplements were much, much worse:
The actual quantity of galantamine in the supplements ranged from less than 2% to 110% of the labeled quantity. Only one product contained galantamine within 10% of the label. Three of the ten products tested were also contaminated, with Bacillus cereus sensu stricto– enterotoxin genes.
Here’s the product-by-product breakdown:
Drug vs. Supplement matters – but should it?
This small study showed that galantamine, when sold as a generic drug, was accurately labeled and free from contamination. This is not the case with galantamine sold as dietary supplements, where the consumer may be purchasing contaminated products that contain a fraction of the galantamine promised.
The dietary supplement industry has exploded in size over the past few decades, with much of that credit owed to the lack of regulatory oversight. But it’s left a marketplace where consumers cannot trust that what is on the label is accurate, or that their supplement was manufactured to high quality standards. This double-standard has made it harder, rather than easier, for consumers to use supplements safely. Until a single, rigorous standard is applied across all consumer health products, there will continue to be uncertainty and even safety risks for users of dietary supplements.
We benefit from regulation, if only to ensure we’re getting what we’re paying for. Weak regulation that doesn’t require manufacturers to demonstrate quality before products are sold, or fails to hold manufacturers accountable for quality, might be expected to result in poor-quality products. And that’s the reality we see today.

