Depending on where you live, and the persistence of public health rules like masking and vaccination entry requirements, it feels like the beginning of the end of COVID-19, or maybe the end of the end. While some have made the decision to “move on” after more than two years, there’s still a lot of COVID-19 being spread around, along with the continued problem of getting populations vaccinated. Recognizing that disease control will continued to be challenged by new variants, waning immunity, possible vaccine escape, and continued vaccine hesitancy (where vaccines are widely available), we continue to need good measures for preventing or controlling disease.
A year into the pandemic I blogged about one of the earliest randomized controlled trials that studied vitamin D for the prevention of COVID-19. Vitamin D has long been thought to play a role in supporting immune function, in addition to effects on muscle and bone. Given many people live where there is inadequate sunlight to support vitamin D synthesis for parts of the year, deficiency, particularly in older adults, is common. There has been widespread interest in the possible role of vitamin D supplements to help raise vitamin D levels, and possibly prevent infection or minimize the severity of disease. This was the case prior to the arrival of COVID-19, and interest grew in the early period of the pandemic, particularly before the arrival of effective vaccines.
Trials studying the effectiveness of vitamin D for the prevention of upper respiratory infections have been either negative or weakly positive. No clear, consistent benefit has been established. A challenge with much of the observational data is that vitamin D blood levels may be a indicator of poor overall health status, but deficiency may not independently be causing more illness. This highlights the importance of prospective trials with randomization, where actual causality can be inferred. The study I blogged about a year ago was an interesting design of a single dose of 200,000 IU (which would be the equivalent of taking 200 tablets of the standard 1000 IU tablet strength). Compared to placebo, the massive dose did raise vitamin D levels in the blood but had no effect on any of the outcomes measured.
The trial
This new trial, which has been published as a preprint (meaning it has not undergone peer review), was recently discussed on Twitter by epidemiologist Gideon Meyerowitz-Katz. It is from David A. Jolliffe and colleagues from a number of centres across the United Kingdom. It’s titled “Vitamin D Supplements for Prevention of Covid-19 or other Acute Respiratory Infections: a Phase 3 Randomized Controlled Trial (CORONAVIT)”. CORONAVIT was a prospective trial conducted “within” the COVIDENCE cohort study, which was a trial established in May 2020 with the goal of identifying risk factors related to COVID-19 infections.
This was a three-arm, parallel, open label study. To be included, you had to be 16 years of age and participating in the COVIDENCE trial. You were excluded if you were already taking vitamin D, or had a small number of medical conditions. 6,200 patients were assessed as eligible, and randomized (1:1:2) as follows:
- High Dose Group – Offered supply of 3,200 IU vitamin D per day if their blood 25(OH)D levels were less than 75 nmol/L
- Low Dose Group – Offered supply of 800 IU vitamin D per day if their blood 25(OH)D levels were less than 75 nmol/L
- No Dose Group – Not offered testing or any supply of vitamin D
Participants were asked to complete monthly follow-up survey which included questions about respiratory infections, exacerbations of asthma or chronic obstructive pulmonary disease, COVID-19 symptoms, and adverse effects. The final survey was sent in June 2021 which was the six-month follow-up. Vitamin D testing was offered to a subset of participants (800 in each treatment group and 400 who were randomized but received no treatment.)
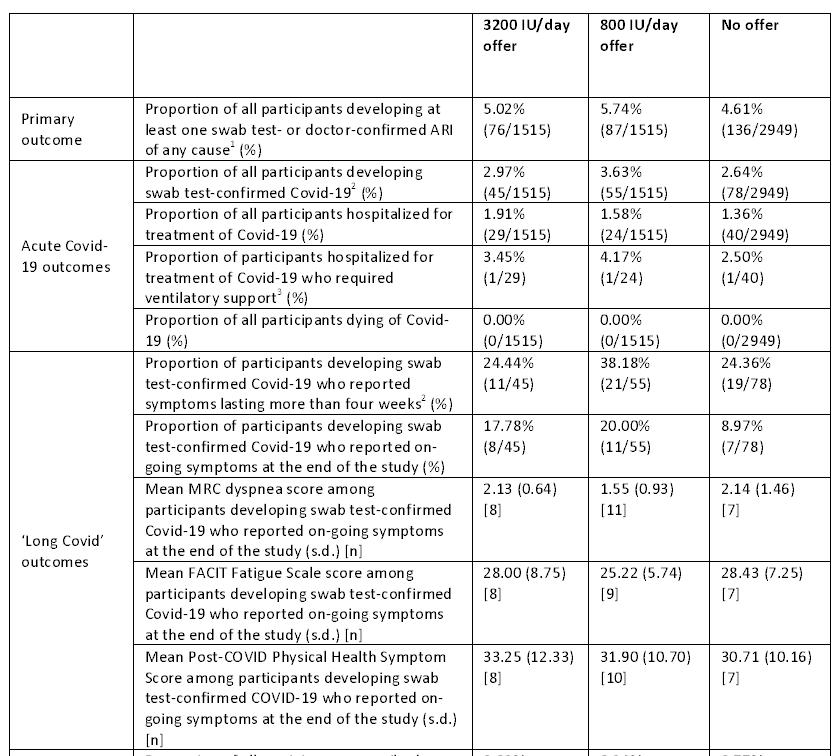
The primary endpoint was the proportion of participants that developed at least one (swab- or physician-confirmed) acute respiratory illness of any kinds. Secondary endpoints included:
- the proportion of participants developing (PCR-confirmed) COVID-19
- the proportion of participants hospitalized for COVID-19
- the proportion of hospitalized patients requiring respiratory support (i.e., mechanical ventilation)
- the proportion of all participants dying of COVID-19
- the proportion of participants developing confirmed COVID-19 who reported symptoms lasting more than four weeks
- the proportion reporting ongoing symptoms at the end of the study
Harms that were measured included the incidence of death, serious adverse effects (and the % leading to discontinuation from the study), high calcium and high levels of vitamin D.
The results
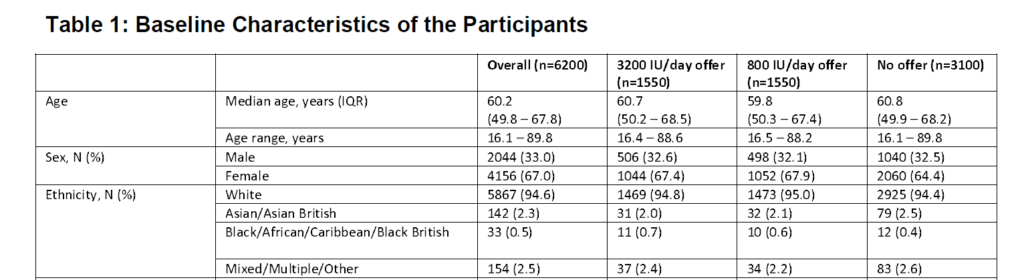
Here is a snapshot from Table 1 showing baseline characteristics. The median age of participants was 60 with a fairly wide age range (16-88+), predominantly female, and overwhelmingly White (94%+).
Only a small percentage (2.5%) of participants had been vaccinated (1 or more doses) at the time of recruitment.
In those where baseline vitamin D was tested, the mean 25(OH)D was 39.7nmol/L. Per the UK National Health Service, adequate levels are defined as ≥75 nmol/L, and 97.4% were below this threshold. Almost all (95.4%) of participants randomized to the treatment groups consented to a 25(OH)D test (by mail).
Follow-up was for six months (December 2020 to June 2021). During this period almost 90% received one or more doses of a COVID-19 vaccine. 90.9% of participants randomized to treatment reported they took their vitamin D supplement at least 6 days per week. In the intention-to treat analysis, there were significant increases in vitamin D levels noted in those on the supplements:
- High dose treatment 102.9 nmol/L
- Lower dose treatment 79.4 nmol/L
- No treatment 66.6 nmol/L
In a sensitivity analysis where non-adherent participants were excluded as well as those in the no-treatment group that actually took vitamin D supplements, the differences were greater:
- High dose treatment 103.4 nmol/L
- Lower dose treatment 79.5 nmol/L
- No treatment 53.7 nmol/L
Primary and secondary endpoints
So did vitamin D prevent respiratory infections, including COVID-19? No. There were no statistically significant differences between the group in terms of the proportion who experienced at least one confirmed acute respiratory infection. With some of the measures like symptom persistence, the vitamin D groups look (non-significantly) worse. There were also no differences in the incidence or severity of COVID-19 infections, the duration of related symptoms, or in any other measure (I have trimmed out the P values here for readability, they’re on page 20 if you want to see them).
A subgroup analysis showed no evidence that vaccination modified the effects of the allocation. Moreover, the sensitivity analysis (described above) did not reveal any differences compared to the primary, intention-to-treat, analysis. That is, excluding patients in the control group who took vitamin D supplements didn’t change the results. Also, excluding patients in the treatment groups who were non-compliant with therapy didn’t change the results.
There were few adverse events. A few patients in the high-dose group developed hypercalcemia and symptoms resolved with the discontinuation of vitamin D.
Conclusion: Vitamin D strikes out again
There were multiple strengths to this study, including the baseline low levels of vitamin D, and the demonstrable effectiveness of the supplements. Compared to the study I blogged about a year ago, this was a more typical (daily) dose versus a single massive dose. There were few dropouts and a modest number of COVID-19 cases. The limited number of cases, particularly of hospitalizations, means we need to be cautious in making definitive conclusions. However, there was no suggestion of benefit.
In this randomized controlled trial, vitamin D had no effects on the incidence or consequences of COVID-19 or other acute respiratory infections. This was despite active treatment with vitamin D that did meaningfully raise serum vitamin D levels.
This is another prospective trial with vitamin D in COVID-19 to show no meaningful effect in the prevention of acute illness. Having said that, it’s clear that (at least in this population) vitamin D deficiency is widespread, and supplementation may be appropriate for individuals anyway. But there continues to be no persuasive evidence to suggest that vitamin D supplementation will help protect you from COVID-19 infections.