In rosuvastatin should we trust?
People love the idea of preventive medicine. Preventing a disease, before it occurs, seems intuitively obvious. But when it comes to taking medicine to prevent a disease before it occurs, people tend to be much less comfortable. Not only are there the concerns about the “medicalization” of healthy people, there are good questions about benefits, risks, and costs. Cardiovascular disease will kill many of us, so there’s been decades of research studying how to prevent that first heart attack or stroke. But even if you’re born with good genes and do everything possible to prevent heart disease (e.g., don’t smoke, exercise regularly, eat a healthy diet, moderate your alcohol, and keep your weight down) you’re still at risk of heart disease. And if you have one or more risk factors for disease, your lifetime risk goes up dramatically. Once you’ve had your first heart attack or stroke, the effectiveness of medical therapy is clear. Drug therapy with medication like the “statins” class of cholesterol-lowering drugs reduces deaths from cardiovascular disease. Given their unambiguous effectiveness, and the high likelihood that many of us will eventually have cardiovascular disease of some sort, the idea of “pre-treating” otherwise-healthy people with drug therapy to possibly prevent that first event has been held out as a potential public health strategy. There’s new evidence that tests this hypothesis, and the results are surprising.
One polypill to medicate them all?
Preventing a disease before it occurs is called primary prevention. So-called “lifestyle” approaches to primary prevention of cardiovascular disease are always the preferred approach: Changing behaviors may be low cost, are simple, and can be safe. Smoking control efforts are probably the best example of such an approach – reduce population levels of smoking, and you’ll see decreases in cardiovascular disease. Medications have been proposed as a secondary approach to disease prevention. The polypill concept has been around for some time. The idea is to bundle one or more low-dose medications into a single pill, with the objective of changing lifetime risk factors. In someone that’s otherwise well, convincing them to take multiple medications, daily, for a lifetime is not likely to be acceptable to many. The polypill could theoretically prevent cardiovascular disease through multiple methods (e.g., by targeting blood pressure and/or blood lipids), and (it’s hoped) reduce overall risks. One of the key ideas of the polypill concept is that it should not require close monitoring that is burdensome to patients and physicians. The patient should be able to take it daily and essentially go about their life as they always have. The more monitoring, and the greater the side effects, the lower the likelihood that anyone is going to be willing to take any medication for a lifetime, especially when they’re not actually sick yet.
If we were going to build a polypill, we’d probably look for drugs with a track record of reducing deaths in cardiovascular disease. Drug like aspirin (ASA), the statins, beta-blockers, and hypertension drugs like ACE inhibitors and angiotensin receptor blockers (ARBs) would be likely candidates, as they reduce the risk of death in people who have already had their first heart attack or stroke. Last week there was a widely-reported study of a polypill published. The trial, called “HOPE-3”, examined the use of statins and blood pressure drugs to prevent heart disease. This massive study of 12,705 people was co-sponsored by a pharmaceutical manufacturer (Astrazeneca, makers of Crestor) and the Canadian Institutes of Health Research, the primary government funder of health research in Canada.
What did the HOPE-3 trial try to answer?
HOPE-3 was a clinical trial designed to test the real-world effectiveness of a polypill. It studied a diverse population (29% Chinese, 29% Hispanic, 20% white, 20% South Asian or other Asian, 2% black, and 2% “other”.) This was a double-blind, placebo-controlled trial that randomized 12,705 people into one of four groups: rosuvastatin 10mg+placebo, rosuvastatin 10mg+candesartan 16mg & hydrochlorothiazide (HCTZ) 10mg, candesartan & HCTZ + placebo, or placebo+placebo. This slide, via the investigators, illustrates:
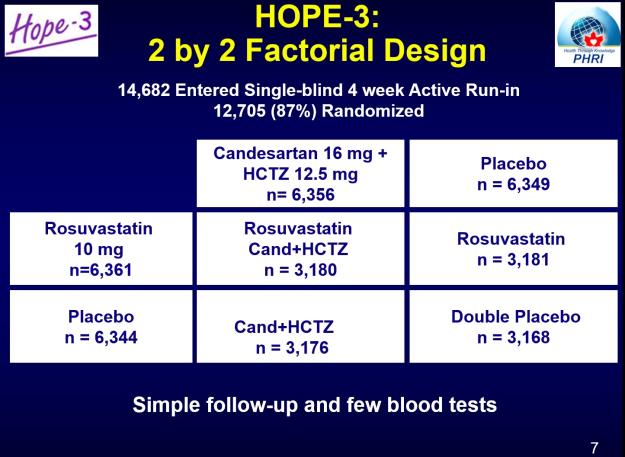
This design allowed the individual evaluation of the cholesterol-lowering “statin” drug, the blood pressure drugs, and the combination of three drugs. The trial was designed to mimic “real life” as much as possible. Entry in the trial wasn’t based on blood pressure or lipids. There were no dose adjustments after you started the trial. Men had to be aged 55 years of age or older, and women, 65 years of age or older. Participants had to be free of cardiovascular disease but have at least one risk factor (besides their age) such as:
- An increased waist-to-hip ratio
- Smoker
- Low HDL (the “good” cholesterol in the blood)
- Dysglycemia (abnormal blood sugar levels)
- Mild kidney dysfunction
- A family history of heart disease
You were excluded from the study if you had clear cardiovascular disease already, if there were medical reasons you couldn’t take the drugs, or if you already had kidney dysfunction or low blood pressure causing symptoms. The study took place in 28 countries – but not the United States, interestingly. The first coprimary outcome was a composite of death due to cardiovascular disease, heart attacks and strokes. The secondary primary endpoint added resuscitated cardiac arrest, heart failure, and revascularization to the first outcome.
Because of the 2×2 design, there are three trials published, and they’re currently available free from the New England Journal of Medicine:
“Cholesterol Lowering in Intermediate-Risk Persons without Cardiovascular Disease” (Lonn et al.)
“Blood Pressure Lowering in Intermediate-Risk Persons without Cardiovascular Disease” (Yusef et al.)
“Blood Pressure and Cholesterol Lowering in Intermediate-Risk Persons without Cardiovascular Disease (Yusef et al.)
Before getting to the results, I want to look at the adherence. Specifically, how many stayed on therapy? Primary prevention is treatment when you’re not ill, so there’s likely to be little real-world acceptance of any medication that people don’t stick with, especially when the treatment is for life. Somewhat surprisingly, for all the complaints that you may hear about the side effects of drugs, these treatments were very well tolerated, with little difference from placebo. Again, a slide from the trial group illustrates:
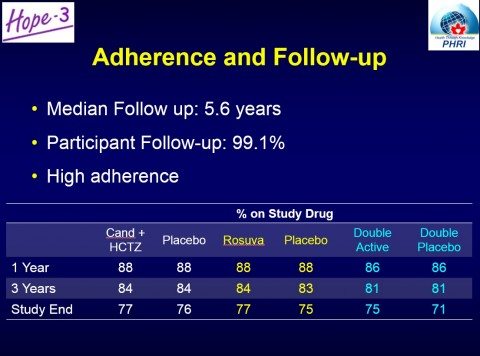
While more people taking statins had muscle pain or weakness (5.8% vs. 4.7%), (classical statin side effects), there was no difference in discontinuation rates for this reason. Overall, the drug therapies were well tolerated, and the results show that more people stayed on triple drug therapy than those on a double placebo. One notable area where there was an excess of harms was in surgery for cataracts, where those taking statins had more surgery than those on placebo (3.8% vs. 3.1%).
Does HOPE-3 give hope?
On to the results, and this is where the study gets really interesting. There are three papers here and I’m not going to recite all the findings. I’ll focus just on the primary endpoint, and you can look at the PDFs above for the others. Treatment with a statin reduced cardiovascular events by a relative 24% with an absolute reduction of 1.1%. Overall, the anti-hypertensives did not reduce the risk of cardiovascular events at all – but there were differences depending on whether or not you had hypertension when you entered the trial. Combined statin + antihypertensive treatment was no better than statin alone. And in all three active treatment groups, there were no differences in “all cause” mortality (i.e., death for any reason).
The study was most clear when it comes to primary prevention with statins: Rosuvastatin offers meaningful benefits to those at intermediate risk of cardiovascular disease. There were fewer deaths and fewer strokes. This finding was independent of the cholesterol levels when patient started the trial. You “set it and forget it”, and the strategy largely worked. Importantly, the results show the benefits of using statins based on risk factors for disease, rather than the traditional approach of looking at lipid levels to guide decisions.
What about blood pressure control? The third of patients with the highest levels of blood pressure did benefit from taking the dual drug therapy. In the middle third, there was no benefit. And in the bottom third, blood pressure lowering caused harm. Some of the criticisms raised have included the low doses of the medication used, the duration of the trial, or the choice to use hydrochlorothiazide instead of chlorthalidone, a similar drug that has better evidence of benefit. It could be a combination of factors. The takeaway from this trial is that (unlike what we learned with statins and cholesterol) if there’s no high blood pressure, then you shouldn’t take medication to lower your blood pressure. In patients with high blood pressure, already on medication, and at intermediate risk of cardiovascular disease, then adding a statin is an option that may be worth considering, as it will add meaningful (though small) benefits over lowering blood pressure alone.
Put statins in the drinking water?
One of the key results from this study is that while statins offer real benefits, those benefits are, from a population perspective, modest. If statins provide a 1% absolute benefit over 5 years, then we must treat 100 people for five years to benefit one person. I calculated the cost in Canada to be about $135 per year for 10mg a day of generic rosuvastatin. I understand that the same drug and dose in the United States, where there’s no generic available, will cost about $3,600 per year. Using the Canadian costs, that means it will cost approximately $67,500 to prevent one major cardiovascular event. It’s estimated that three-quarters of men over the age of 55 and women over the age of 60 could be eligible for statins based on this data. While statins are not tremendously expensive, they are not free, and a decision to put millions on statins means resources not spent in other areas – potentially other measures that improve overall live expectancy, such as focusing on the social determinants of health.
Primary prevention with statins is lifelong therapy. Before undertaking it, one needs to consider the expected benefits (they are real, but modest), and the potential for harms and side effects (relatively small, but not zero). It’s possible (though not proven) that the lifetime benefits of statins may be larger, as the follow-up in this trial was just over 5 years. Perhaps more than any other type of medical treatment, primary prevention mandates a close consideration and respect for a patient’s own preferences. Some may be willing to take medication to reduce the likelihood that they will have a cardiovascular event. Others in the exact same circumstances may opt not to. There is not right or wrong answer. With HOPE-3, we now have good data to inform those discussions.
