Alternative medicine, by definition, consists of medicine that either has not been shown to work or has been shown not to work. To paraphrase an old adage yet again, medicine that has been shown to work with an acceptable risk-benefit ceases to be “alternative” and becomes simply “medicine.”
Unlike the case for many conditions commonly treated with alternative medicine, whether or not a treatment works against cancer is determined by its impact on the hardest of “hard” endpoints: Survival. A patient either survives his cancer or he does not. Even the “softer” endpoints used to assess the effectiveness of cancer treatments tend to be much harder than for most other diseases, such as progression-free survival (the cancer either progresses after treatment or it does not) or recurrence-free survival (a cancer either recurs after treatment eliminates it, or it doesn’t). Yes, although there are lots of other aspects of cancer treatment to be assessed, such as quality of life and adverse reactions, at the very heart of evaluating any treatment for a specific cancer are the questions: Does the therapy save the lives of cancer patients? Does it prolong survival, and, if it does, by how much and at what cost?
One might reasonably predict that, for alternative medicine and any given cancer, the answer to both questions will be no. However, the question is much harder to study than one might guess if you don’t do cancer research yourself. For one thing, it is unethical to do a randomized, controlled clinical trial of a treatment with no evidence of benefit. So, except for very uncommon situations (e.g., the Gonzalez protocol, which was tested in a clinical trial against pancreatic cancer and failed miserably), we have to use other methods to investigate the effect of alternative medicine use on survival in cancer patients. Yes, anecdotes like that of Michaela Jakubczyk-Eckert, who died a horrible potentially preventable death from breast cancer because she chose the quackery of Ryke Geerd Hamer’s German New Medicine and stopped her neoadjuvant chemotherapy, which allowed the tumor to grow back bigger and deadlier than ever, are powerful and very likely representative of what happens, but this is science-based medicine. What are the actual numbers. Yes, I’ve seen at least a dozen women like Ms. Jakubczyk-Eckert through my career, but what is the effect of choosing alternative medicine beyond my clinical experience and in cancers that I personally do not treat?
Such were the thoughts going through my mind as I was made aware through social media of a study published online ahead of print in the Journal of the National Cancer Institute by Skyler et al, entitled “Use of Alternative Medicine for Cancer and Its Impact on Survival.” In it, Skyler B. Johnson, Henry S. Park, Cary P. Gross, James B. Yu, all from the Department of Therapeutic Radiation (basically radiation oncology) at Yale, seek to answer the question: What is the effect of choosing alternative medicine as the primary treatment for a potentially curable cancer on a cancer patient’s chance of surviving his or her disease?
The newest study showing that alternative medicine kills cancer patients
The latest study, by Skylar et al, is a good demonstration of how difficult it is to study alternative medicine use in cancer patients. I’ll show you why in a moment. First, however, the authors introduce why it is so important to study this:
Delay or refusal of conventional cancer treatment (CCT), when done in favor of alternative medicine (AM), may have serious survival implications for cancer patients (1–7). However, there is limited research evaluating the use and effectiveness of AM, partly due to data scarcity or patient hesitance to disclose nonmedical therapy to their providers (8,9). To address this knowledge gap, we used the four most prevalent cancers (breast, prostate, lung, and colorectal) in the United States (10) from the National Cancer Database (NCDB) between 2004 and 2013 to identify the factors associated with AM selection and compared survival outcomes between AM and CCT.
Yes, there is a paucity of studies evaluating the use of alternative medicine in cancer. (I will cite some of the other studies that exist after I discuss this one.) The reason is clear. It’s hard, and data are lacking. This brings me to the National Cancer Database.
There are two very large databases in the US that are commonly mined for cancer outcomes. One, of course, is the Surveillance, Epidemiology, and End Results (SEER) database, which is maintained by the National Cancer Institute. The program began in 1973 and consists of cancer registries all over the country that enter data regarding cancer outcomes in a standardized format, which includes patient demographics, primary tumor site, tumor morphology and stage at diagnosis, first course of treatment, and follow-up for vital status. Mortality and patient survival are tracked, with the mortality data coming from the National Center for Health Statistics and population data coming periodically from the Census Bureau. As large as it is, though, because of many gaps in coverage SEER only reports cancer outcomes for 28% of the US population. Still, it is a large database that’s been around for 45 years. However, working with it in collaboration in the past, I’ve found that it has notable oddities and omissions. Often it is behind the times in tracking important variables, such as HER2 status in breast cancer, which SEER didn’t begin tracking until 2011 or so, even though HER2 status had been used for at least a decade before that.
That’s probably why the authors chose the National Cancer Database, which is a joint project of the American College of Surgeons and the American Cancer Society. It is a clinical oncology database sourced from hospital registry data collected by the more than 1,500 facilities accredited by the American College of Surgeons Commission on Cancer (CoC). Data cover more than 70% of newly diagnosed cancer cases nationwide and are used to develop quality improvement initiatives and set quality standards for cancer care in many hospitals across the US.
Now, imagine that you want to look at the effect of alternative medicine use on cancer mortality, and you had access to a large database like this. How would you go about doing it? There are a lot of things you have to consider. First, you would want to look at potentially curable cancers, because you want to find out if patients with curable cancers who choose alternative medicine die at a much higher rate than those who use conventional therapy. Thus, you have to exclude patients who had metastatic disease at the time of diagnosis. Another important thing you have to do is to choose cancers that have a reasonable rate of cure using conventional therapy. Choosing pancreatic cancer, for instance, wouldn’t make much sense, since the vast majority of pancreatic cancer patients, even those without metastatic disease at diagnosis, die of their disease. Even though we know from the Gonzalez trial that patients with pancreatic cancer still do much worse, dying faster and suffering more, than those treated with conventional medicine, such a difference would be unlikely to show up in a database study like this. So the authors chose four common cancers, nonmetastatic breast, prostate, lung, or colorectal cancer.
Similarly, how do you identify patients in the database who underwent alternative medicine treatment rather than conventional therapy? This is a question that is not as easy to answer as it sounds. For one thing, many databases don’t include that information. One statewide database with which I worked, for instance, didn’t even have a field for alternative medicine (or even “complementary and alternative medicine”), even though it had over 750 elements tracked for each patient. This is almost certainly the reason the SEER database was not used for this study.
Fortunately, the NCDB has data fields that can help:
Patients who underwent AM were identified as those coded as “other-unproven: cancer treatments administered by nonmedical personnel” and who also did not receive CCT, defined as chemotherapy, radiotherapy, surgery, and/or hormone therapy. Patients with metastatic disease at diagnosis, stage IV disease based on the American Joint Commission on Cancer (AJCC) staging system (11), receipt of upfront treatment with palliative intent, and unknown treatment status or clinical or demographic characteristics were excluded.
The authors identified only 280 patients who fit the criteria, and noted that patients in the alternative medicine group were likely to be younger, female, and have a lower Charlson-Deyo Comorbidity Score (CDCS, a measure of preexisting comorbidities or of how “sick” the patient is at the time of diagnosis). In multivariate analyses controlling for clinical and demographic factors, the authors found that patients undergoing alternative cancer treatments were more likely to have breast cancer, higher education, Intermountain West or Pacific regions of residence, stage 2 or 3 disease, and a lower CDCS. All of this jibes with the usual impression that patients who choose alternative cancer cures tend to be of higher socioeconomic status and education, as well as healthier than average.
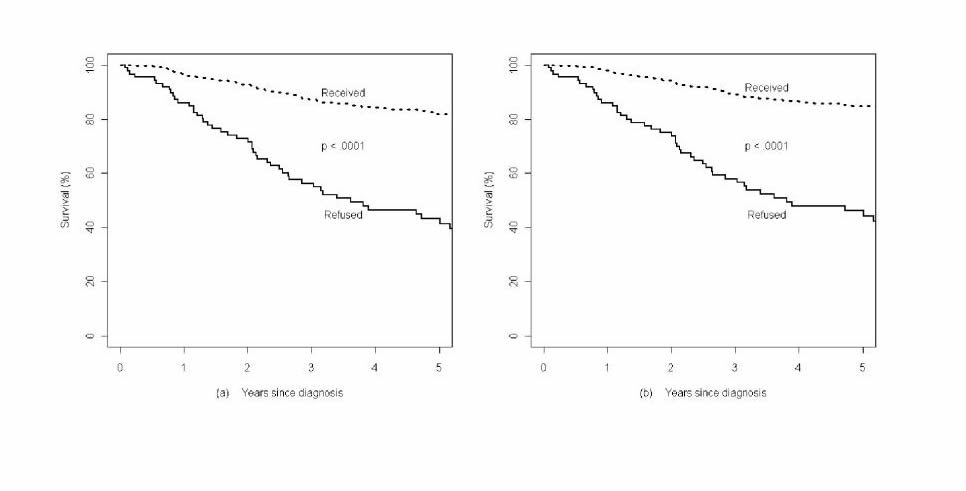
So what were the results? Not surprisingly, the risk of death was higher for three out of the four cancers. Overall, the hazard ratio (HR) for death was 2.5 (95% confidence interval [CI] 1.88 to 3.27); 5.68 for breast cancer (CI 3.22 to 10.04); 2.17 for lung cancer (CI 1.42 to 3.32); and 4.57 for colorectal cancer (CI 1.66 to 12.61). The differences observed were not significant for prostate cancer, likely because the survival with conventional therapy was so high to begin with. Prostate cancer tends to have a long natural course, and in this study numbers were small and follow-up too short.
As the cliché goes, a picture is worth a thousand words. Here are the survival curves:

Survival curves for (A) all patients, (B) breast, (C) prostate, (D) lung, and (E) colorectal cancers.
Obviously, this study has a lot of limitations. For one thing, the use of conventional medicine is likely to have been under-ascertained (i.e., undercounted or incompletely identified). After all, as I’ve discussed with other patients, some of those who choose alternative medicine to treat their cancer ultimately realize that it’s not working and come back to conventional medicine. Such patients could also have gone to different institutions that aren’t covered by the NCDB. However, if such a bias occurred, it would have tended to make the differences in survival between the alternative medicine group and the conventional treatment group smaller, not larger, meaning that if such a bias occurred in this study the harm caused by choosing alternative medicine is likely to be significantly worse than reported.
Other studies
Obviously, this study by Skyler et al is just one study, and the most recent. There are other studies showing similar results, but unfortunately they are relatively few. For example, the first study I remember encountering after I had started blogging about medicine and alternative medicine that addressed the question of the effect of alternative medicine on cancer survival was published in 2006 in the American Journal of Surgery by Chang et al. This study used a different methodology to study the effect of alternative medicine on breast cancer survival. Specifically, the authors did a chart review of patients who refused or delayed recommended treatment of their breast cancer to pursue alternative therapies and compared their survival to that expected in patients with disease of their type and stage.
Even eleven years later, this study remains interesting to me because it’s the first one that I can recall encountering that explicitly looked at the outcomes of patients who chose “alternative” therapies as their primary treatment. There are lots of studies out there looking at alternative medicine use in cancer patients, but these mainly look at patients who use it in addition to conventional therapy (i.e., as “complementary” therapy). This study does have one strength, too, compared to most such studies, in that the patient population comes from a community practice, not an academic medical center. Consequently, it can be viewed as more representative of the “real” world situation than many studies done in academic medical centers, where the patient population may be self-selected as people as either motivated enough to seek out tertiary care centers or sick enough that their community surgeons and oncologists refer them.
One thing that was also rather fascinating about the study was the variety of alternative therapies that the study population opted for, including coral calcium, coenzyme Q10, herbs, dietary therapy, high dose vitamins, mushrooms, chelation therapy, poison hemlock (I’m not kidding), and a variety of unspecified therapies. Because of the sheer variety of therapies used and the low number of patients using each individual therapy, it was not possible to “identify particular alternative modalities that were particularly ineffective,” as the authors put it.
Who says scientific papers don’t occasionally have sarcasm in them?
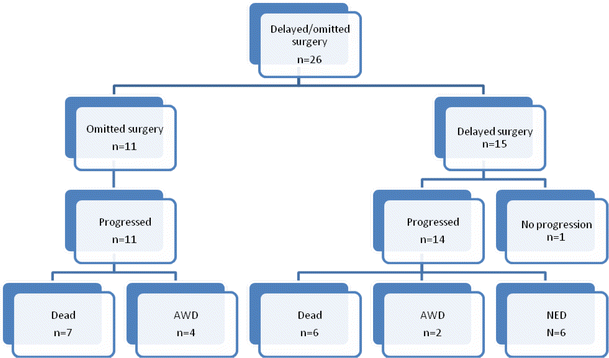
Basically, the study identified 47 breast cancer patients who opted for alternative therapy, but follow-up information was only available for 33. These were divided into patients who refused surgical treatment altogether; patients who delayed appropriate surgical treatment to pursue alternative treatments; patients who refused adequate sampling of the lymph nodes; patients who refused procedures to ensure adequate local control (additional surgery and/or radiation therapy); and patients who refused chemotherapy. I’m going to concentrate first on patients who refused or delayed surgery, for the simple reason that surgery is what is curative for breast cancer and differences in survival due to adjuvant therapy can be as low as the single digit percentages, depending upon the stage of the cancer.
Of patients who refused surgery, none of the six patients identified were Stage IV (metastatic disease) at initial diagnosis. However, five out of these six patients who returned to the surgeons doing the study had progressed to stage IV, with a median time of follow-up of 14 months, with one death within a year. That’s pretty amazing, given that two of these patients were Stage I upon initial presentation. There were also five patients identified who initially refused surgery in favor of alternative medicine, all of whom were Stage II or III. The median time between diagnosis and surgery was 37 months. All five demonstrated progression of their disease, with three progressing to Stage IV disease and one of these dying of metastatic disease. Thus, 10/11 patients who refused surgery experienced significant disease progression, with 8/11 of these progressing to stage IV disease, which is incurable, and 2/11 dying within the short time frame of the study.
Not surprisingly, patients who declined chemotherapy or hormonal therapy fared better because, as I’ve explained before, for operable breast cancer, the single most efficacious intervention is surgery, and it is not that uncommon for patients with even fairly large tumors to be “cured” with surgery alone. Indeed, the benefits of chemotherapy are fairly modest in many cases, particularly those with early stage disease. In a small number of patients, it was difficult to quantify the effect of choosing alternative medicine over conventional chemotherapy, but the authors were able to estimate that the relative risk of death in 10 years in those who refused chemotherapy was 1.54; i.e., a 54% higher chance of dying within 10 years compared to those treated with conventional medicine.
A few years later, there was followup study published in the Annals of Surgical Oncology examining the same question, this time with 61 patients to study and ten year follow-ups available. Again, a retrospective chart review was performed, with telephone interviews conducted when possible. Again, authors calculated an estimated expected 10-year survival rate and/or 10-year relapse rate of each patient if they used recommended therapy and compared it to what was actually observed in the alternative medicine group. For patients who delayed surgery, the prognosis at initial presentation was compared with the prognosis based upon return presentation.
The results were just as grim. As before, patients were divided into two groups, those who refused or delayed surgery (n=26) and those who refused adjuvant therapy, such as radiation and chemotherapy (n=35). In the group that refused surgery, 96.2% of patients experienced progression of their cancer, and 50% died of their disease. The mean stage at diagnosis in this group was II. The mean stage when patients in this group re-presented after primary treatment with alternative medicine was IV, which is, again, incurable. In the group refusing adjuvant therapy, progression occurred in 86.2% of those in the ASG, and 20% died of disease. Overall, in the surgery group, the expected mean 10-year survival calculated for those omitting surgery was 69.5%. In comparison the actual observed 10-year survival for these patients was 36.4% at a median follow-up of 33 months. For the patients who delayed surgery to undertake alternative treatments, the figures were 73.6% expected 10-year survival versus a 60% observed 10-year survival.
The authors also noted that, for the patients refusing adjuvant chemotherapy or hormonal therapy, the median tumor size at presentation was 2 cm and that the mean calculated 10-year relapse-free survival at initial presentation was 59.2%. Using a commonly utilized online tool to calculate the benefit of chemotherapy based on aggregated clinical trials, the authors noted that, had recommended adjuvant therapy been followed, relapse-free survival would have improved to 74.3%. However, the observed relapse-free survival was only 13.8%. They also noted that, although the patients’ intent was to avoid traditional therapy, ultimately, 6 patients in this group started endocrine therapy to control breast cancer recurrence, and 21 had salvage chemotherapy to attempt to control recurrent disease.
Here’s a summary of the patients who refused surgery (AWD = “alive with disease; NED = “no evaluable disease,” or basically no detectable disease):
You get the idea. This is far worse than what would be expected in patients undergoing standard treatment. As before, this study shows that refusing surgery results in the worst outcomes, which is something that has been known for a long time. For instance, this 2005 study utilizing data from the Geneva Cancer Registry. This study did not look at alternative treatments but rather at just the refusal of patients to undergo surgery for their breast cancer. The results were very similar to what the other studies I discussed showed:
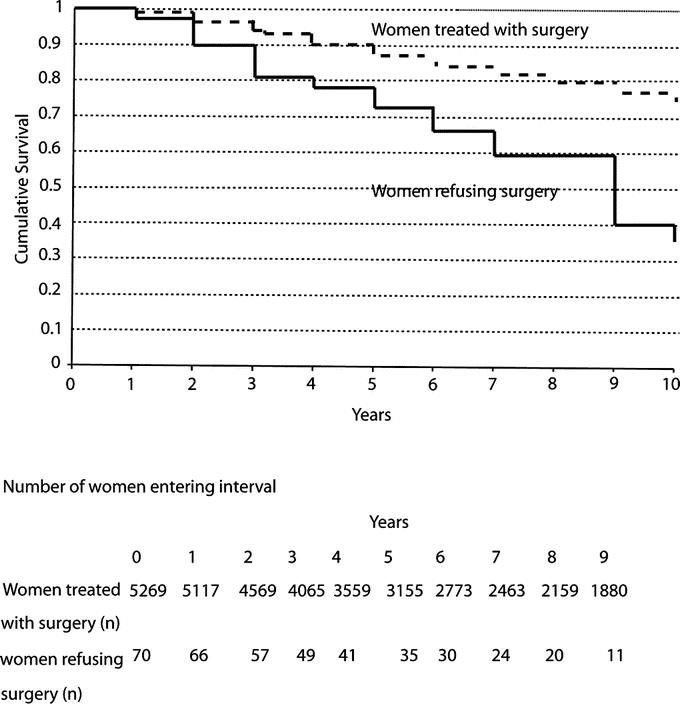
Another study, this time published in the World Journal of Surgery in 2012 examined women in the Northern Alberta Health Region who declined recommended primary standard treatments and included 185 women who refused standard treatment, resulting in a median delay in instituting effective treatment of up to 101 months. The survival graphs look depressingly the same:
Both Scott Gavura and I discussed this study in detail when it was published. Of note, quoth the authors:
Our data showed that almost all the patients who initially refused treatment progressed to a higher stage on later presentation at the cancer center. The majority of the patients (57%) in our series initially chose CAM as the primary treatment instead of surgery. Those who had chosen CAM had disease progression with particularly poor disease-specific survival when compared to those who received standard treatment.
Finally, a recent study from Malaysia found a strong correlation between CAM use and delays in diagnosis and treatment in breast cancer patients, although this study also suggested that the reason many women use alternative medicine is because they don’t have good access to high quality medical care.
As an aside, I will note that one tendency in some of these studies that drives me up a wall is the authors’ tendency to refer to alternative medicine used as primary treatment for a cancer “CAM.” It is not CAM. CAM, by definition (you know, the “complementary” in “complementary and alternative medicine”) is not used as primary treatment for cancer or anything else. If an unproven or ineffective treatment “outside the mainstream” is being used to treat a cancer, it’s not CAM. It’s alternative medicine. I don’t like the term “CAM,” because it was designed as a means to slip unproven treatments into conventional medicine by adding them to conventional therapy when they are unnecessary, but it is the language we have.
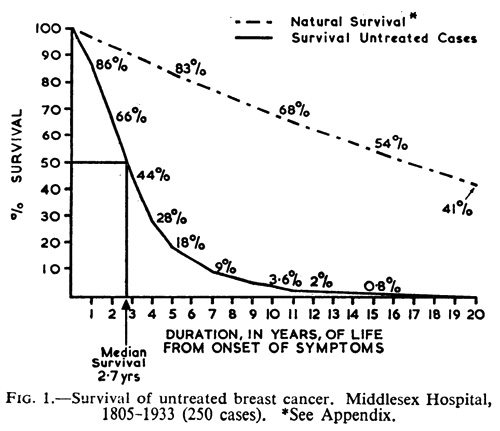
Finally, compare the curves above to this curve, which I use frequently. This is from a famous study from 1962 by Bloom and Richardson that calculated the survival of patients with untreated breast cancer. It was carried out long before mammographic screening became the norm, which means that the cancers were detected by palpation and other clinical signs. The point is that survival in untreated breast cancer is longer than you might guess:
The authors compared their data with previous studies from the 1920s and 1930s and found that their curve lined up very closely with previous data on expected survival in a group of women, all comers, with untreated breast cancer. Basically, the median survival was 2.7 years. 18% of the patients survived five years; 3.6% survived 10 years; and 0.8% survived 15 years. Of note, it was 19 years before all 250 patients in the study were dead. Notice how the curves above for women choosing alternative medicine over conventional therapy get closer to resembling the curve from the Bloom-Richardson paper. The don’t quite get there because we’re in a different era, where breast cancers are usually discovered by mammography and were much smaller at diagnosis than in the patients in that paper.
But what about CAM?
I made a point of expressing annoyance over how some authors throw around the term “CAM” when they mean “alternative medicine.” This brings up a question. Does CAM use affect cancer survival? Again, there is a paucity of evidence. We might expect that it probably doesn’t, at least when used as the strict, “integrative medicine”-approved definition of unconventional therapies used in addition to conventional medicine is followed. Again, there aren’t a lot of studies, and most of those seek to demonstrate that a specific set of CAM involving “mind-body” or psychotherapeutic interventions actually improve survival in cancer patients. Unfortunately, they do not, as has been discussed here before.
Other studies have looked at CAM in cancer patients, with mixed and mostly unfavorable results. For example, a study from Norway found a modest negative effect of CAM use in cancers, with a higher death rate over the study period (79% versus 65%), with a hazard ratio for death of 1.30 that just missed statistical significance, leading the authors to conclude that the use of alternative medicine “seems to predict a shorter survival from cancer” and that “the effect appears predominantly in patients with a good performance status” (i.e., the healthiest patients). In addition, a Korean study of terminally ill cancer patients assessed the use of CAM on survival and health-related quality of life (HRQOL), finding that CAM did not provide a survival benefit but did negatively impact various measures that go into calculating HRQOL.
Finally, a large prospective trial known as the Health Eating, Activity, and Lifestyle (HEAL) Study examined 707 patients with stage I to IIIA breast cancer. No associations between CAM use and breast cancer-specific or total mortality were observed. Another study, a pooled analysis from 2012 of four studies conducted in Hawaii in 1994–2003 and linked to the Hawaii Tumor Registry to obtain long-term follow-up information, also found no overall link between CAM use and breast cancer mortality, but did find links between the use of energy medicine use and death as well as finding that Filipino women who used CAM were at a higher risk of death. Those results might have been spurious, but I note that the data did actually show a trend towards a correlation between overall CAM use and death that just didn’t reach statistical significance.
What’s probably most problematic about CAM is not so much that using it with conventional medicine harms patients. Rather, it’s the mindset that leads to strong associations between CAM use and, for instance, refusing adjuvant chemotherapy, as I have discussed in detail before and noted by way of citing a Malaysian study in which CAM use was associated with delays in diagnosis.
The bottom line: Alternative medicine kills cancer patients
There is a surprising paucity of evidence regarding whether the use of CAM in addition to conventional therapy has an adverse effect on cancer survival, but the evidence that we do have is very clear on at least one thing: CAM does not improve cancer survival. Less clear is whether CAM has an adverse effect on cancer survival. There the evidence is conflicting, but there is plenty of reason to be concerned about the use of CAM in cancer, given correlations between CAM use and delays in diagnosis and refusal of adjuvant chemotherapy.
Regardless of what you think of the phenomenon of “integrative” medicine or CAM, there is one thing that the existing medical literature, as thin as it is, indicates, and it’s that alternative medicine kills cancer patients. It is basically no different than refusing treatment altogether and much more expensive and troublesome. Given that there is no good evidence of specific anticancer effects from close to all (if not all) alternative medicines, there was never any reason to suspect that the answer would be otherwise. Moreover, as strongly suggested by the Gonzalez trial, alternative medicine use as a primary therapy for cancer often means that patients aren’t receiving effective, science-based supportive care for their cancers, resulting in inadequate (or nonexistent) relief of cancer-related symptoms and unnecessary suffering. Use of alternative medicine alone to treat cancer is likely to be a death sentence, or at least to cause delays that make ultimate cancer treatment with conventional medicine more difficult and less likely to be successful.