Science-Based Medicine authors tend to focus attention on practices that misuse or ignore the tools of science. This is not a criticism. As a daily reader and occasional contributor, I recognize that shining a light on pseudoscience is a critical part SBM’s mission. It is what brings me back day after day. Sometimes, however, it is nice to highlight what real science and real evidence can do.
In my world of treating patients with retinal disease, a revolution has taken place over the past few years. The most aggressive form of macular degeneration has been transformed from a relentlessly progressive, disabling disease to one which can be tamed with medication. Now, patients diagnosed with exudative macular degeneration can expect stabilization and even improvement in vision.
It is a story worthy of a Hollywood movie. Start with a reluctant hero; add controversy, Wall Street, politics, and most important of all, a happy ending.
Background: Age-related macular degeneration
Age related macular degeneration (ARMD) is the leading cause of irreversible legal blindness among adults in the industrialized world. In the United States nearly 2 million people are estimated to have more advanced forms of macular degeneration. But this is just the tip of the iceberg. Over 7 million Americans have earlier forms of ARMD with lesser degrees of vision loss and a lifelong risk of progression to advanced disease. These numbers are expected to rise dramatically as the population ages.
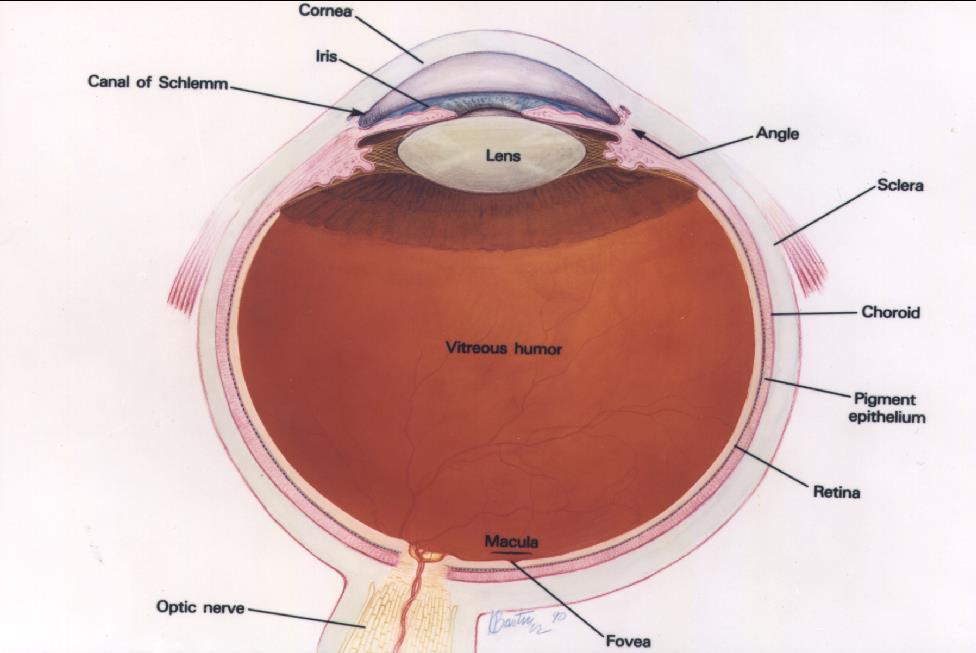
Courtesy of the National Eye Institute, National Institutes of Health
The retina lines the inner wall of the eye like wallpaper, and functions like the film in a camera. The cornea and lens, in the front of the eye, focus light on the retina. The brain interprets the signals from the retina as vision. The macula is the anatomic and physiologic center of the retina. It is, quite literally, the center of your visual world. It is responsible for the sharpest, most detailed vision and needs to be healthy for reading and other detailed visual tasks.
ARMD occurs in two forms, or stages, referred to as “dry” and “wet”. Most patients have the dry type. In the early stages, patients usually have no symptoms, and the lucky ones will never experience noticeable vision loss. Over time, there is often slow anatomic and functional deterioration of the macula. Patients may experience a variety of symptoms including, blurriness, loss of contrast sensitivity, distortion, and blind spots in or near the center of their vision, but the progression tends to be quite gradual, over many years.
Wet (or exudative) disease is the most feared form of ARMD. Only a minority of patients develop wet ARMD, but those that do often experience a rapid, catastrophic loss of vision over a period of weeks or months. The defining event in transformation from dry to wet ARMD is the growth of abnormal blood vessels within the macula. These blood vessels cause bleeding, swelling, and scarring.

Image as seen with normal vision. Image courtesy of the National Eye Institute, National Institutes of Health

Simulated vision for a patients with macular degeneration. Image courtesy of the National Eye Institute, National Institutes of Health
Patients with ARMD comprise a large part of my practice. They are typically in or approaching their retirement years. As the disease progresses they experience loss of activities that give them pleasure such as reading, watching television, and using the computer. They also face loss of activities that give them independence such as driving and handling their own financial affairs.
Additional educational material about macular degeneration can be found here, and here.
Treatments for wet ARMD have evolved over the decades. First there was laser treatment. This strategy used laser energy to coagulate or “burn” the abnormal blood vessels. The laser created a lot of collateral damage and resulted in blind spots in the patient’s vision, but these tended to be more stable the than damage created by the growing blood vessels. Only a small minority of patients were eligible for this treatment, and the rate of recurrence was quite high.
The next treatment was photodynamic therapy or “cold laser”. This treatment consisted of the administration of an intravenous photosensitizing dye, followed by low intensity laser. This combination caused much less collateral damage than a traditional laser, but the results were far from spectacular. On the average, treatment slowed down, but did not halt the progressive vision loss. Only a small minority of patients experienced improvement in vision. Furthermore, certain large subsets of patients did not respond favorably to treatment at all.
A reluctant hero, and a new era
The hero of our story is a physician/researcher named Judah Folkman. David Gorski wrote an appropriately admiring obituary when Dr. Folkman died in 2008. If you are not familiar with Dr. Folkman, and want an in-depth biography of a consummate practitioner of science-based medicine, read his story.
Dr. Folkman was a bit of a maverick. He chose to create a new field of research, a field that was not taken very seriously by his contemporaries, at least not at the beginning of his career. His attitude was maverick, but his work ethic was not. He started from scratch, by creating and refining his own models. He worked meticulously, behaved humbly, and spoke cautiously. He was a mentor to many young investigators who passed through his laboratory, and an inspiration to countless others.
Dr. Folkman did not set out to create a treatment for macular degeneration. It is doubtful that this disease was even on his radar when he launched his research career. His goal was to find new treatments for cancer. Folkman recognized that solid tumors required a blood supply to grow. He reasoned that if he could understand how tumors recruited new blood vessels, he might be able to use that knowledge to treat cancer. His early work was met with much skepticism. Eventually his work was recognized and emulated. The field became known as “angiogenesis”. Folkman’s lab became an incubator for young investigators to launch their own careers.
Much of angiogenesis research was identifying biologic compounds that stimulated the growth of new blood vessels, and also searching for others that stopped new vessels from growing. Because the eye contains avascular structures, it was used as a model for experiments in Dr. Folkman’s lab. Like cancer, macular degeneration (and several other blinding eye diseases) resulted from unwanted blood vessels growing in the wrong place at the wrong time. Researchers in eye disease recognized the relevance of angiogenesis research to eye disease, and several launched their careers in Dr. Folkman’s laboratory.
It wasn’t just academics who jumped on the angiogenesis bandwagon. Pharmaceutical companies recognized the potential for drugs that could inhibit the growth of blood vessels. The main target was cancer, but the possibilities treating eye diseases did not escape some of the companies.
In 1989 scientists at the biotech company Genentech isolated a family of compounds that appeared to be critical in how the body (and cancers) regulate the growth of new blood vessels. They became known as Vascular Endothelial Growth Factor (VEGF). In later research (by several investigators), it was discovered that VEGF is produced in the human eye, and is found at higher concentrations in diseases for which new blood vessels grew. In animal models, VEGF was both necessary and sufficient for neovascularization to occur. It was a reasonable hypothesis that if the action of VEGF could be blocked, the growth of new blood vessels in diseases like wet ARMD could be arrested.
The race is on
The race was on to develop a drug targeting VEGF for eye diseases. The first company to successfully develop such a drug was a start-up called Eyetech. The first disease they studied was ARMD. The drug was called pegaptanib, later sold as Macugen. Pegaptanib is from a class of compounds known as aptamers. Aptamers function much like antibodies. Antibodies are made of proteins, aptamers are made of nucleic acids. Pegaptanib was designed to bind to VEGF. The idea was that pegaptanib would act like a sponge, and soak up VEGF so it could not bind to its receptors, and thus neovascularization could be arrested.
Eyetech navigated clinical trials rather quickly and the studies were favorable. Patients treated with Macugen ended up with better vision than controls. FDA approved the drug in December 2004.
Although Macugen was a huge step forward, and established proof of principle that VEGF blockade was a valid strategy in the treatment of ARMD, the result were not the “home run” we had hoped for. Like photodynamic therapy, Macugen slowed down the progression of wet ARMD, but like photodymanic therapy, the trend was still for progressive loss of vision. Only a small proportion of patients actually enjoyed an improvement in vision.
Meanwhile, the scientists at the biotech company Genentech were working on their own VEGF-binding drug for ARMD. Their drug, ranibizumab was an antibody fragment. Antibodies are proteins that are part of the immune system. Two regions of each antibody bind to foreign material such as bacteria, viruses, or other molecules. Antibodies are fairly specific regarding the materials to which they bind. Genentech eliminated much of the non-binding portions of an antibody directed against VEGF. By making the molecule smaller, they hoped that the drug would be better able to penetrate into the tissues of the eye.
Even in phase 1 and 2 studies, ranibizumab showed great promise. The phase 3 data were finally presented at a meeting in July, 2005. The results were impressive. There were two phase 3 studies, each treating a different subset of wet ARMD patients. In both studies, patients treated with ranibizumab fared better than controls; not just a little better, but much better. For the first time, the average patient’s vision improved by a significant amount. Very few patients lost vision.
The table displays data from the phase 3 study comparing ranibizumab (Lucentis) to photodynamic therapy (PDT). Points on the chart represent the mean change in vision over the 24 months of the study. Note that patients treated with PDT (the usual care for patients when this study was done) lost vision over the course of the study. Patients treated with Lucentis had sustained improvements in vision. The average patient treated with Lucentis ended up able to read letters less than half the size of the average patient treated with PDT.
Patients would have to wait one more year for Lucentis to be approved by the FDA.
You’re going to do what to my eye?
So far, I haven’t mentioned the way anti-VEGF drugs like Macugen and Lucentis are given. The technique is called “intravitreal injection.” The vitreous cavity is a space that occupies about 4/5 the volume of the eye. It is filled with a transparent gel called vitreous. It has been known for many years that many drugs can be injected safely into the vitreous cavity via hypodermic syringe and a very fine needle. The vitreous acts as somewhat of a drug reservoir. There are advantages to intravitreal injection. It can be difficult to achieve therapeutic drug levels in the eye through conventional routes. By injecting the drug into the eye, high concentrations can be achieved in the target tissues, with minimal exposure or toxicity to the rest of the body. Although it sounds barbaric, patients seem to tolerate these injections very well. It is routinely done in the office, and most retina specialists now do dozens of these injections each week. If you are not squeamish about such things here is a video of an intravitreal injection.
In the clinical trials, patients treated with Macugen received injections every 6 weeks. For Lucentis, injections were given every month. That’s right, patients submitted themselves to an experimental needle in the eye eight to twelve times per year. In the real world, strategies are being explored to try to reduce the number of injections necessary to achieve good results, but repeated injections are still required for most patients.
The shot heard ’round the world
In July 2005, at the very same meeting at which the phase 3 ranibizumab data were presented, was another paper which changed the world. Phil Rosenfeld, MD, PhD, a well-known and respected physician from the University of Miami, presented a case series of patients he had treated with intravitreal injections of bevacizumab (Avastin). The case series was small, and uncontrolled, but the results were intriguing. The drug appeared to work, and work well, even in patients who had failed to respond to other therapies. Bevacizumab is an anti-VEGF antibody which had been FDA-approved to treat colon cancer. It was also a product of Genentech and sold under the name Avastin. Avastin was actually the parent compound of Lucentis. Most of the nonbinding parts of the Avastin antibody were removed to create Lucentis.
What happened over the next few months is legendary. Doctors and patients had seen the phenomenal data for Lucentis. The drug vastly outperformed all known treatments; but Lucentis was not yet FDA-approved, and thus unavailable to them. The data for intravitreal Avastin were tantalizing. A few doctors went home and tried using Avastin as an off-label treatment for their patients. The results surpassed anything they had ever used. Word spread quickly. More and more physicians tried Avastin, and liked the results they were seeing. The treatment became quite prevalent world-wide.
Sticker shock!
Lucentis was finally FDA approved in 2006, nearly 1 year after the data were presented. The wholesale price: $1,995/dose. Remember, in the clinical trials Lucentis was dosed monthly. Some patients needed injections in both eyes! Medicare will generally pay 80% of the cost of drugs administered in doctors’ offices. Unless the patient had good supplemental insurance to pick up the other 20%, they carried a significant personal financial burden.
In contrast, Avastin was cheap. Although fairly pricey as an intravenous treatment for cancer, the amount of drug needed for an intravitreal injection was so small that each dose was inexpensive. Compounding pharmacies were willing to dose out the drug in pre-filled syringes for a small fee. Even with these added costs, each dose of Avastin cost approximately $50.
Based on the solid data of safety and efficacy, and the stamp of approval from the FDA, many physicians adopted the use of Lucentis. Other doctors had become quite comfortable using Avastin and saw no reason to switch. This was fueled, in no small part, to anger over the high price of Lucentis. Although Avastin and Lucentis had not yet been compared in a head-to-head trial, many doctors perceived that the two drugs were equally effective, and felt it was irresponsible to use the one that was 40 times the cost of the other.
Corporate shenanigans
The situation was unprecedented. Genentech had created Lucentis, a spectacularly effective and expensive drug for wet ARMD. This was a win for patients and stockholders, but sales were undercut by competition from a cheap, off-label competitor. Ironically, the cheap competition was their own drug, Avastin. The availability of cheap Avastin significantly impacted the sales of Lucentis. The great Avastin vs. Lucentis debate was born. One was more likely to read news about macular degeneration treatments in the business section of the newspaper than the science or medicine sections.
The New York Times reported a secret program instituted by Genentech that provided financial incentives to high-volume prescribers of Lucentis. The contract physicians signed to participate in the program stipulated that the agreement was confidential.
Genentech also announced their intent to stop selling Avastin to compounding pharmacies, a move that would shut down access of most physicians (and patients) to the less-expensive option. Genentech said the move was out of concern for the safety of patients. Ultimately, they backed down from their decision under heavy pressure from professional organizations of ophthalmologists.
The showdown
The Avastin vs. Lucentis debate went on for many years. The financial ramifications were of importance to patients as well as Medicare and other payers. The economics of the standoff were summarized by Phil Rosenfeld (the same man who introduced the world to intravireal Avastin) in testimony before the Senate Special Committee on Aging. There were physicians firmly entrenched on each side. Surveys and Medicare records showed that use of Avastin was slightly more prevalent than Lucentis by a ratio of approximately 6:4. Lucentis users had the backing of high-quality randomized clinical trials, and the imprimatur of the FDA. Avastin users did not have the highest quality studies to back up their choice, but they were confident that their treatments were as safe and effective as Lucentis and fiscally more responsible.
It was clear that a head-to-head Avastin vs. Lucentis study was needed. The National Eye Institute (NEI) of the National Institutes of Health decided to sponsor such a study, titled: “Comparison of Age-Related Macular Degeneration Treatments Trials” (CATT). The coordination of all the government agencies responsible for overseeing and paying for the study proved daunting. Putting the regulatory and financial puzzle pieces together delayed the start of the study for over a year. The leadership of the study expressed their frustration negotiating the political minefield in an editorial. It makes for interesting reading.
And the winner isn’t…
The one and two year results of the CATT study were published in 2011 and 2012, respectively. The overall conclusion was that, for all practical purposes, Avastin and Lucentis were equally effective. But, there was a catch; there were an increased number of adverse events in the group treated with Avastin.
When given intravenously in cancer studies, VEGF-blocking drugs cause hypertension, and increase the risk of thromboembolic events (strokes and heart attacks). The small doses given in the eye were felt to be at low risk. However, after intravitreal injection, the systemic exposure to Avastin is slightly higher than Lucentis, theoretically imparting a greater risk of thromboembolic events. It turned out there were no differences between the drugs for mortality or thromboembolic events. The excess adverse events in the Avastin group did not follow an easily identifiable pattern. The meaning of these safety data are the subject of much debate and further investigation.
A new kid on the block
Even before the final Avastin vs. Lucentis data were announced, the world became more interesting and more complicated. A new drug, aflibercept, was approved by the FDA to treat macular degeneration in November 2011. This was brought to market by another biotech company, Regeneron. The drug is sold as “Eylea”. In the pivotal phase 3 trials, Eylea was compared to Lucentis. For the first 3 months, Eylea was dosed every month, just like Lucentis. After the third dose Eylea was given every other month. With this reduced dosing schedule, patients treated with Eylea did just as well as patients treated with monthly Lucentis.
The price-tag for Eylea: $1850/dose, less expensive per dose than Lucentis, potentially with fewer doses. Even with the reduced dosing schedule it is still far more expensive than Avastin.
Epilogue
The availability of anti-VEGF drugs for exudative macular degeneration has completely transformed the expectations of patients with wet ARMD. Patients who have developed wet macular degeneration within the past 8 years are much more likely to be able to read, drive, knit, paint, watch TV, golf, write a check, and recognize their grandchildren, than those who preceded them.
But that is not the end of the story. These drugs are also proving effective for the treatment of a growing list of eye diseases including one of the greatest scourges to good vision: diabetic retinopathy. Stay tuned.

