One of the things that feels the weirdest about having done the same job, having been in the same specialty, for a longer and longer time is that you frequently feel, as the late, great Yogi Berra would have put it, déjà vu all over again. This is particularly true in science and medicine, where the same issues come up again and again and again, often with the same arguments on either side. Sometimes the same players are even involved. So it is with mammography recommendations. Indeed, I’m feeling déjà vu all over again right now, as I read headlines like “Women advised to get mammograms later, less often“, “American Cancer Society, in a Shift, Recommends Fewer Mammograms“, and “ACS: Breast cancer screening should begin at age 45“. What provoked these headlines was a major revision in the American Cancer Society’s recommendation for mammographic screening for breast cancer in women at average risk of the disease. In a seeming replay from 2009, when the United States Preventative Services Taskforce (USPSTF) sent shockwaves through the breast cancer world by recommending that most women not start mammography until age 50 and then only to have it done every two years instead of every year, the American Cancer Society (ACS) has now just similarly ratcheted back its recommendations for screening mammography, just not as much as the USPSTF did. The new recommendations were communicated in a special communication published by JAMA on Tuesday.
What changed regarding mammography recommendations
Before we get to the issues, how, specifically, did the ACS change its mammography recommendations? Before this change, the ACS basically recommended the same thing that most other American professional societies dealing with breast cancer did: yearly mammography starting at age 40 for the rest of a woman’s life. The new guidelines now recommend that women with an average risk of breast cancer should undergo regular screening mammography starting at age 45 years and continuing annually until age 54. From age 55 and older, the ACS recommends that women transition to every two years. (More details below.) As I Tweeted when I saw these recommendations, basically it appears that the ACS has more or less split the difference between the old recommendations and the USPSTF recommendations.
So why is the ACS changing its recommendations? And what does this say about the science and our values regarding cancer screening? If you’ve been reading this blog, you know that over the last several years there has been a steady drip, drip, drip of studies that range from highlighting the downside of widespread mammographic screening to downright questioning the value of mammography. That’s why I’ve been discussing rethinking screening for breast cancer since at least 2008. Basically, you can go back and read my old posts and, if you have a lot of time and are enough of a glutton for punishment to read them all, watch the evolution of my thinking about breast cancer screening over the last seven years.
Back in the day, I used to fully support breast cancer screening beginning at age 40 and proceeding yearly throughout. As I examined more and more of the evidence, I became less enthusiastic about screening so intensely and started to believe that starting at 40 was too young for most women. Indeed, I was probably the only breast cancer doctor at my cancer center in 2009 who supported the USPSTF recommendations when they were announced, which led to some—shall we say?—interesting discussions about what should be said to the press and what a press release our cancer center wanted to release ASAP should actually say. I also got myself into a little…trouble…for criticizing colleagues in radiology—not from my institution, I hasten to add!—for some rather blatant turf protection. Let’s just say that a prominent radiologist, one who’s achieved far more renown in his field than I ever have in mine, was most displeased with some of my commentary and let me know about it. I found this displeasure odd, given that I am most definitely not a nihilist with respect to mammography screening (and, make no mistake, there are quite a few of those out there these days). I’m just a lot more balanced and aware of its limitations than I used to be. On the other hand, I did call him out for some of his more obnoxious comments that implied that those who question mammography are cackling gleefully at the thought of more women dying of breast cancer. Interestingly, I don’t seem to get asked to contribute to such press releases that much anymore, but in fairness neither do most of the other breast cancer clinicians I work with; so I probably can’t blame it on my previous outspokenness.
What brought me to this point is an increasing understanding of the concepts of overdiagnosis and lead time bias, coupled with a string of studies that show more modest benefits (and, in one case, no benefit) from screening mammography. To be honest, the attack dog reaction by some mammography supporters to some of these negative studies also set my skeptical antennae a’twitchin’ as well.
Overdiagnosis: Driving change in screening guidelines
So what is overdiagnosis? I’ve discussed it many times before, but it’s a concept relevant to any program in which masses of asymptomatic people are screened for a disease with a test. I like to boil the concept down to the idea that the harder you look for something (in this case, a disease), the more of it you will find. The problem is that some of what you find will be disease that either won’t progress or is so slow-progressing that it would never threaten the life of the patient or cause her major problems. In essence, such discovered disease does not need treatment, but it’s treated anyway if discovered by screening. It has to be, because, unless doctors have a way of determining whether a disease will progress, they’re morally obligated to treat it as though it will progress. This is true for breast cancer. Indeed, there is evidence that as many as one in three mammography-detected breast cancers are overdiagnosed and that as many as one in five can spontaneously regress. Granted, other estimates are lower which adds a large degree of uncertainty to these discussions, but it is clear that overdiagnosis is a major problem, radiology societies’ tendency to deny it notwithstanding.
Nowhere is the problem of overdiagnosis of breast cancer as acute as it is for the entity known as ductal carcinoma in situ (DCIS). DCIS is basically defined as cancerous cells in the breast ducts that have not yet broken out of the ducts to invade the surrounding breast tissue. As I’ve discussed before, it’s not clear whether we should consider DCIS cancer, precancerous, or something else. It’s clear that a significant percentage of DCIS will ultimately progress to breast cancer, but we don’t yet have reliable tests to determine which DCIS will and which will not progress to cancer. Here’s where the overdiagnosis comes in. Back before the 1970s, DCIS used to be a very uncommon diagnosis because by the time DCIS forms a mass there is usually invasive cancer in it as well. However, between the 1970s and 2000s, the incidence of DCIS increased by more than 16-fold. Does anyone think that the incidence of DCIS really increased that much in such a short period of time, a mere three decades? Of course not. We’re just finding a lot more of it. Unfortunately, a significant fraction of it is overdiagnosed and, inevitably, overtreated, even though a recent study has called into question our aggressive treatment of DCIS.
Another issue that confounds the correlation between finding cancer early and decreased mortality is lead time bias. Basically, lead time bias is a phenomenon in which the early detection of cancer leads to an apparent increase in cancer survival even if early detection has no effect whatsoever in the progression of the disease. Because disease is detected earlier, the time from detection to when the disease would have become symptomatic is added to the time from symptoms to death to produce this inflated survival rate. It takes careful study design to tease out whether an improvement in survival is due to lead time bias or a real treatment effect. For more information, read this classic brief explanation of this phenomenon. Another problem is length bias, which is the tendency of screening tests to detect slowly progressing (and therefore likely less aggressive) disease while still asymptomatic. Sharon Begley summarizes the difficulties thusly:
It turns out that some cancers detected by mammograms would never have posed a threat to a woman’s health or life. Others are so slow-growing that even if they’re not detected until they cause symptoms, they’re treatable. Still others are so aggressive that even catching them “early” is too late. In these situations, mammograms therefore have little effect on whether a woman will die of breast cancer.
The ACS recommendations
With these confounders in mind, let’s take a look at the ACS analysis, which is was published in JAMA in the form of a communication laying out the guidelines and their rationale, a systematic review of the benefits and harms of mammography, and an accompanying editorial. The systematic review found that screening was associated with a reduction in breast cancer mortality of around 20%, which is consistent with a lot of previous data. Basically, the ACS incorporated standards recommended by the Institute of Medicine into its guidelines development protocol “to ensure a more trustworthy, transparent, and consistent process for developing and communicating guidelines.” It selected the Duke University Evidence Synthesis Group to conduct an independent systematic evidence review of the breast cancer screening literature in response to a request for proposals, a process it referred to as the evidence review. In addition, the ACS commissioned the Breast Cancer Surveillance Consortium (BCSC) to update previously published analyses of screening intervals and outcomes. The methods were described thusly:
In accordance with the new guideline development process, the ACS organized an interdisciplinary guideline development group (GDG) consisting of clinicians (n = 4), biostatisticians (n = 2), epidemiologists (n = 2), an economist (n = 1), and patient representatives (n = 2). The GDG developed 5 key questions using the general approach of specifying populations, interventions, comparisons, outcomes, timing of outcomes, and settings (PICOTS) for each question. After evaluating available methods to grade the evidence and the strength of recommendations, the GDG selected the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) system. GRADE is an accepted approach with a defined analytic framework, an explicit consideration of values and preferences in addressing patient-centered outcomes, the capacity for flexibility in evaluating results from observational studies, and separation between quality of evidence and strength of recommendation.
Prior to the ACS submitting its final guidelines for publication, it had them externally reviewed by 26 relevant outside organizations and 22 expert advisors.
For purposes of the review, the ACS considered “average risk” to mean (1) women without a personal history of breast cancer; (2) without a confirmed or suspected mutation that increases the risk of breast cancer (e.g. BRCA1); or (3) a history of previous radiation therapy to the chest at a young age. Studies examined included:
- Controlled studies, including RCTs, pooled patient-level meta-analyses, systematic reviews, and study-level meta-analyses.
- Observational studies (prospective and retrospective cohort studies, incidence-based mortality studies, case-control studies, or cross-sectional studies) published since 2000 that included 1000 or more average-risk women.
- Modeling/simulation studies, because these studies may be the only way to generate estimates of long-term outcomes associated with screening that are not adequately addressed by the RCTs or using modern technology and protocols.
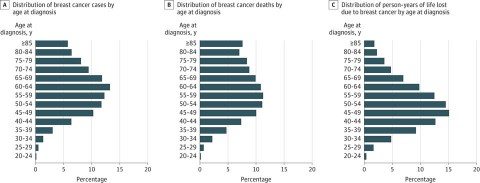
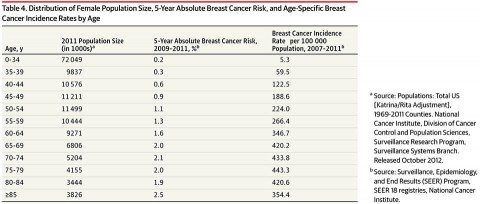
The key innovation in the approach used by the ACS is that, for purposes of estimating risk-benefit ratios, it broke down breast cancer risk not into ten year age intervals, but five year intervals instead. I’ve explained time and time again that the benefits of screening depend upon the prevalence of a disease being screened for in the population being screened. The lower the prevalence, the more likely screening will result in false positives and overdiagnosis. Also, the lower the prevalence of the disease, the fewer women screened who will potentially benefit. For young women, this is particularly true. Here is the relevant age distribution for breast cancer (click to embiggen):
And this is the breast cancer incidence by five-year age groups (click to embiggen):
As you can see, for women below the age of 35 breast cancer risk is very low but rapidly climbs with age. It’s also a continuous curve, but recommendations usually have to be concrete and pick a cutoff age (or ages). As I like to explain whenever the topic of mammography comes up, why not screen beginning at age 20? Why not make that, or 30, the cutoff age to begin screening in average risk women? This table shows why. The risk of breast cancer is so low at age 20 that in essence virtually all findings will be false positives or overdiagnosis. That’s why not even the most outspoken advocates of mammography recommend screening 20 years olds—or even 30 year olds. Obviously, when we get to the 40-50 year age range is when we enter the gray area.
In any case, the ACS ranked its recommendations thusly, with “strong” meaning the group reached consensus that the benefit of the intervention outweighs the harm and “qualified” meaning that there is clear evidence of benefit but less certainty about the balance of risks and benefits. These are the results:
- Women with an average risk of breast cancer should undergo regular screening mammography starting at age 45 years. (Strong Recommendation); 1a. Women aged 45 to 54 years should be screened annually. (Qualified Recommendation); 1b. Women 55 years and older should transition to biennial screening or have the opportunity to continue screening annually. (Qualified Recommendation); 1c. Women should have the opportunity to begin annual screening between the ages of 40 and 44 years. (Qualified Recommendation)
- Women should continue screening mammography as long as their overall health is good and they have a life expectancy of 10 years or longer. (Qualified Recommendation)
- The ACS does not recommend clinical breast examination for breast cancer screening among average-risk women at any age. (Qualified Recommendation)
These recommendations are qualified by a further recommendation that women “should have the opportunity to begin annual screening between the ages of 40 and 44 years,” presumably if that’s what they and/or their doctors want and that women 55 and older who want to keep screening annually should be able to, again, if that’s what they and their doctors want. Overall, as I mentioned above, to me these recommendations come across as “splitting the difference” between the USPSTF recommendations and older recommendations.
One surprising recommendation is the ACS statement that clinical breast examinations (CBE) are not recommended for average-risk women as a qualified recommendation. Here’s the rationale:
Previous guideline recommendations for routine CBE have acknowledged the limitations in evidence. For Key Question 3, the evidence review found a lack of evidence showing any benefit of a CBE alone or in conjunction with screening mammography. There is moderate-quality evidence that adding CBE to mammography screening increased the false-positive rate. No studies were identified assessing other critical outcomes. A supplemental search identified studies on CBE performance characteristics, most of which show that the addition of CBE will detect a small number of additional breast cancers (ie, 2%-6%) compared with mammography alone.93- 95 There are no data on whether patient outcomes are improved with CBE. Given the lack of benefit concurrent with the increase in false-positive rates, CBE is not recommended for breast cancer screening among average-risk, asymptomatic women at any age. Recognizing the time constraints in a typical clinic visit, clinicians should use this time instead for ascertaining family history and counseling women regarding the importance of being alert to breast changes and the potential benefits, limitations, and harms of screening mammography.
This recommendation would not affect my practice, because by definition any woman referred to me is suspected of having breast disease, either benign or malignant; so a clinical examination is warranted. It is clearly directed more at primary care physicians, who, after all, do the vast majority of breast cancer screening and refer patients with positive findings to the relevant specialists. Nor would this apply to women who have had breast cancer and are undergoing follow-up after treatment.
Putting things in perspective
Whenever looking at screening guidelines, I always find that it helps to put things in perspective by looking at absolute numbers rather than percentages. Sure, it’s great to say that mammography results in a 15-20% reduction in breast cancer mortality, but what does that really mean? In the accompanying editorial, Nancy L. Keating and Lydia E. Pace point out:
As clinicians embark on shared decision making about breast cancer screening with their patients, several key messages are worth highlighting. First, the vast majority of women who are diagnosed with breast cancer will do well regardless of whether their cancer was found by mammography. For women in their 40s and 50s, randomized trial evidence suggests that screening mammography modestly decreases breast cancer mortality by approximately 15%. Although more recent observational studies may suggest a larger benefit, given the methodologic limitations of such studies, it seems that the randomized trial estimates, while imperfect, are likely to be most accurate. Thus, about 85% of women in their 40s and 50s who die of breast cancer would have died regardless of mammography screening. More sophisticated screening tests that confer a greater reduction in breast cancer mortality would likely decrease breast cancer mortality much more than expanding screening mammography for women in their 40s and 50s.
Moreover, because the risk of breast cancer is low for women in their 40s and to some extent women in their 50s, the modest relative benefit of 15% translates to a very small absolute benefit (approximately 5 of 10 000 women in their 40s and 10 of 10 000 women in their 50s are likely to have a breast cancer death prevented by regular mammography).24 The absolute benefit will be higher for women with a higher absolute risk of breast cancer, underscoring the importance of identifying higher-risk women. Especially for average-risk women, decisions to undergo regular mammography screening must also consider the harms of mammography—most notably the possibility of overdiagnosis and resultant overtreatment (age-specific estimates of which are lacking) and also the risks of false positives and unnecessary biopsies (known to be greater in younger women and women screened more frequently).
As the JAMA article noted, overdiagnosis is the most severe harm caused by screening, because it results in overtreatment; i.e., women undergoing surgery, radiation, and sometimes even chemotherapy for a condition that would not endanger their lives. Unfortunately, estimates of overdiagnosis vary wildly, from 5% to as high as 50% of screen-detected cancers being overdiagnosed. Personally, my interpretation of the existing literature leads me to see the percentage of screen-diagnosed breast cancers that are overdiagnosed converging around 15-20%, but even that number has a lot of uncertainty. Indeed, even nearly 40 years since the advent of mass screening programs for breast cancer utilizing mammography, it is frustrating to note how much uncertainty remains over the benefits of mammography. One thing is for sure. Before the USPSTF recommendations were first announced, the risks of mammography were rarely discussed in the lay press. Now a more balanced discussion is taking place, and I consider this a good thing. It’s paternalistic to tell patients to undergo yearly screening beginning at 40 without giving them as clear an idea as we can of the risks and benefits of doing so compared to other options, such as starting at age 50 and/or screening less frequently.
The backlash
If there’s one thing I’ve noticed since 2009, it’s that the criticism of studies that find less benefit and/or more harm due to mammography than previously believed seems to be less intense than what greeted the USPSTF recommendations, which exploded like the proverbial bombshell in the national consciousness when they were announced and provoked harsh resistance from radiologists. At least this time around with the ACS recommendations, I have yet to see a radiologist claiming that we’ll be killing women, the way several prominent ones did in 2009. That’s not to say that they haven’t been critical:
Radiologists, who administer mammograms, blasted the new guidelines. “If a woman wants to reduce, as much as possible, her risk of dying of breast cancer, she will choose yearly mammography starting at age 40,” Dr. Debra Monticciolo of the American College of Radiology said in a statement.
As before, the American College of Radiology regurgitated its same old talking points, such as this:
While ACS states that transitioning to biennial screening is an option for older women, they note that either one or two year intervals would be appropriate as a woman ages. The ACR and SBI strongly encourage women to obtain the maximum lifesaving benefits from mammography by continuing to get annual screening.
If the ACS is correct (and I think it is) and screening every one or two years doesn’t matter after menopause, then what, I ask, are the “maximum lifesaving benefits” of mammography done every year in this age range?
Then there’s this:
“The ACS has strongly reaffirmed that mammography screening saves lives. The new ACS guidelines show that if a woman wants to reduce, as much as possible, her risk of dying of breast cancer, she will choose yearly mammography starting at age 40. A recent study in the British Medical Journal confirms this, showing that early detection of breast cancer is critical for improving breast cancer survival, regardless of therapy advances. Moving away from annual screening of women ages 40 and older puts women’s lives at risk,” said Debra Monticciolo, MD, FACR, chair of the American College of Radiology Breast Imaging Commission.
That particular BMJ study was an observational study that examined the Netherlands Cancer Registry for 173,797 breast cancer patients diagnosed between 1999 and 2012, subdivided into two time cohorts on the basis of breast cancer diagnosis: 1999-2005 and 2006-12. Not surprisingly, the study found that stage at diagnosis is still, even in this age of more effective chemotherapy, strongly correlated with survival: the higher the stage, the worse survival. Of course, as large a study as this was, its result was still about as “Well, duh!” a result as I can imagine, and the study mentions virtually nothing about mammographic screening or the potential effect of lead time bias that could contribute to apparent longer survival. In other words, it does not show that mammography decreases breast cancer mortality.
Finally, as usual, the ACR claims that estimates of overdiagnosis are “vastly overinflated due to key methodological flaws in studies,” while laying down howlers like this:
- The overdiagnosis range is likely 1-10 percent and is principally due to ductal carcinoma in situ (DCIS)
- Very few invasive cancers are over-diagnosed
- NO evidence shows that an invasive cancer has ever gone away or shrank without treatment.
One notes that no studies are provided to support the claim that overdiagnosis is between 1%-10%. I also note that 10% would still be high, and the ACR seemingly allows for the possibility that overdiagnosis is that high. While it is probably true that most overdiagnosis is due to the detection of DCIS, I also note that there is considerable morbidity due to the overtreatment of women with DCIS. Doesn’t the ACR think that’s important, even if DCIS is unlikely to result in death? Women not-uncommonly undergo mastectomies for DCIS, and a recent study suggests that this intensive treatment does not decrease mortality from breast cancer. Finally, while it is true that there is no direct evidence showing that an invasive cancer has ever gone away, there is plenty of circumstantial evidence to show that some invasive cancers either do not progress or even regress.
Not content to use bad scientific arguments, the ACR even resorts to an anecdote of a woman who got her first mammogram at age 38. Of course, this wasn’t exactly a screening mammogram, as the woman was not really asymptomatic. She was concerned about pain and a bruise after being bumped by her child at a water park. The bruise concerned her enough that she made an appointment with her doctor, who was concerned enough to order a mammogram, in part because the woman was nearing 40. The mammogram found a cancer. Of course, we can all find outliers like this. I could point to women from my own practice over the last decade and a half who religiously got their mammograms every year and still were diagnosed with aggressive, nasty breast cancers. Anecdotes appeal to emotions; to make population-level decisions, we require as much objective data as possible.
Data alone, however, are not enough.
Mammographic screening: A value judgment
I criticize the ACR a lot for it history of bad arguments against any study that questions mammography or any new guidelines that suggest that we can do as well detecting cancer with less harm by starting screening later and/or doing it less frequently, and, yes, I do sometimes think there is an element of turf protection going on there. I also believe that these radiologists genuinely believe that they are doing great good. In other words, leaving aside turf, they value detection of breast cancer so much that the potential harms of mass mammographic screening are either downplayed or are considered to be a price worth paying for decreasing breast cancer mortality. I and others who have changed our minds about the intensity of mammographic screening, value reducing harm more, given the minuscule additional benefit that comes from more intensive screening compared to less intensive screening, and the price paid for that tiny additional benefit.
I’ve often said that the question of screening women for breast cancer is not just a scientific question. Science can provide estimates of sensitivity, specificity, the odds of decreasing death from breast cancer, the chances of harm through overdiagnosis and overtreatment, the risk of false positives, and other numbers needed to evaluate the usefulness of screening. What we in the medical profession and society do with these numbers is much more a matter of values than it is of science. Some advocate doing everything possible to decrease death from breast cancer; others are more sanguine about the potential harm in that approach. It all comes down to what we value and, yes, what we are willing to pay for as a society. It’s a debate that’s now been going on for decades. If you want to understand why I say I’m feeling déjà vu all over again, just read this NYT article from 1992 on whether screening should start at age 40 or 50.
Feeling it yet?
In terms of specific medical recommendations, how, exactly, do we balance the potential harms of a screening program like mammography with its known benefits? The ACS guidelines are just the latest attempt to answer that question. Indeed, both the ACS and USPSTF guidelines interpret a vast pool of messy evidence, emphasizing some pieces more than others and coming to slightly different conclusions. Their conclusions are actually remarkably similar to one another but still subject to considerable uncertainty. What individual patients do with these recommendations is a subject of shared decision-making between a woman and her doctor.