Shouldn’t you know that the pills you are paying for are safe, and actually do something?
This week’s post will revisit a topic I recently covered, but it’s time-sensitive and needs your input. Health Canada, the Canadian equivalent to the US Food and Drugs Administration, is considering revisions to the way in which it regulates dietary supplements, which are called “natural health products” in Canada. It is rare that a regulator acknowledges that a regulatory system isn’t working, and publicly expresses a commitment to being more science-based. There is a time-limited opportunity for the public (including all of you non-Canadians!) to provide comment on how supplement regulation could be more closely aligned around scientific principles, rather than the supplement industry’s priorities. Whether you take dietary supplements or not, we can probably all agree that consumers should have access to safe products as well as credible, relevant information about these products, in order to make informed health decisions. It will likely not surprise you that these ideas are seen as threats to supplement manufacturers, who benefit from little regulatory oversight and few restrictions on what can currently be claimed about any product’s effectiveness. Since my last post, there have been some new reactions to the consultation that are worth discussing.
What’s the problem in Canada?
Regular readers to the blog, especially the American readers, will likely be familiar with the Dietary Supplement Health and Education Act of 1994 (DSHEA). DSHEA was an amendment to the U.S. Federal Food, Drug and Cosmetic Act that established the regulatory framework for dietary supplements. It effectively excludes manufacturers of these products from virtually all regulations that are in place for prescription and over-the-counter drugs. DSHEA attempts to distinguish supplements from drugs in a puzzling way, even going as far as defining therapeutic and pharmacologically-active products (e.g., herbs, botanicals, some hormones) to be categorized as foods, and therefore eligible for DSHEA exemptions from the FDA’s drug regulations. Manufacturers can put virtually any claim on a supplement, without any requirement to provide persuasive clinical evidence, as long as it’s accompanied by the Quack Miranda Warning: “These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease.” Disease treatment claims are not permitted, but are typically restated as permissible “structure/function” claims, implying an ability to improve the structure or function of the body. There’s a lack of prospective manufacturing oversight of these products, and there are no effectively no pre-marketing requirements. The FDA can only block the sale dietary supplements only after significant problems have been identified. DSHEA overall has been a win for manufacturers, and for sales, but a disaster for giving consumers quality products, who today cannot trust that what’s on the label is actually in the bottle, or that the products they buy are safe or effective.
When Canada decided to regulate supplements, it took a slightly different path. Instead of calling products “foods” or “drugs” it created a new category: so-called “natural health products”. This category includes nutritional supplements, probiotics, traditional Chinese medicine, vitamins, herbal products, and homeopathy – many of the same products that would be considered “dietary supplements” under DSHEA. These regulations were a compromise: they introduced some manufacturing quality and safety standards, while effectively eliminating the requirement to provide clinical evidence to substantiate efficacy claims. But in Canada, all products are assessed and approved by the regulator. Today, homeopathy is a “natural health product” that is “approved” by Health Canada. While homeopathy is effectively an elaborate placebo system, Health Canada assigns distinct registration numbers, dosages, and even “recommended use” to hundreds of physically indistinguishable brands of sugar pills.
What’s in the consultation?
Health Canada has a nice summary of what the consultation is about:
Health Canada has different ways of overseeing the safety, efficacy, and quality of cosmetics, natural health products, and non-prescription drugs. By safety, we mean that the product will not be harmful or toxic when you use it according to the directions and warnings on the label. Efficacy refers to what the product is meant to do and is usually represented by a claim, e.g., “relieves headache”. A health claim is a description on the product about what it does in relation to someone’s health. Finally, quality means that the product is manufactured under controlled conditions and will be made properly.
While all of these products fall under one law in Canada – the Food and Drugs Act – they are regulated under three separate sets of regulations:
- Cosmetics sold in Canada must meet the Cosmetic Regulations;
- Natural health products sold in Canada must meet the Natural Health Products Regulations; and
- Non-prescription drugs sold in Canada must meet the Food and Drug Regulations.
Regulations, along with related policy and guidance documents, set the rules that companies have to follow so that their products can be sold in Canada. These rules set out how products are evaluated and approved by Health Canada (including what claims can be made); how products should be manufactured, labelled and packaged; how safety and compliance will be monitored once products are on the market; and, what the consequences are for companies if they do not follow the rules.
Even though all three sets of regulations fall under the same Act, they do not set out the same rules for bringing self-care products to market. For example, not all self-care products currently require that the company provide scientific evidence to Health Canada to support the claims made on the label. Similarly, some products undergo a lot of scrutiny before coming to market, while others do not. This means that products could appear almost identical on a store shelf, even though the rules for bringing them to market are quite different. For a consumer weighing the options between similar-looking products, both claiming to treat the same symptom or condition, the differences in the approval process are not apparent. This means that consumers may be choosing a product without the information required to make an informed choice.
Health Canada is examining new approaches to the regulation of self-care products, including refocusing the approval of health claims to those based on scientific proof. A new approach will continue to allow for a wide range of products to be available for Canadian consumers, while, at the same time, taking a more consistent approach to the oversight of self-care products that will ultimately better support consumers’ ability to make an informed decision.
Emphasis added.
Health Canada is proposing regulations based on risk. Low risk products would be subject to few regulatory requirements, and high-risk products would receive a more rigorous review. Risk will be determined by (1) the underlying safety of the product, and (2) the consequences if a product fails to do what it claims. Whether or not a product is “natural” will have no bearing on this assessment. This image illustrates how the scrutiny and regulation could vary:
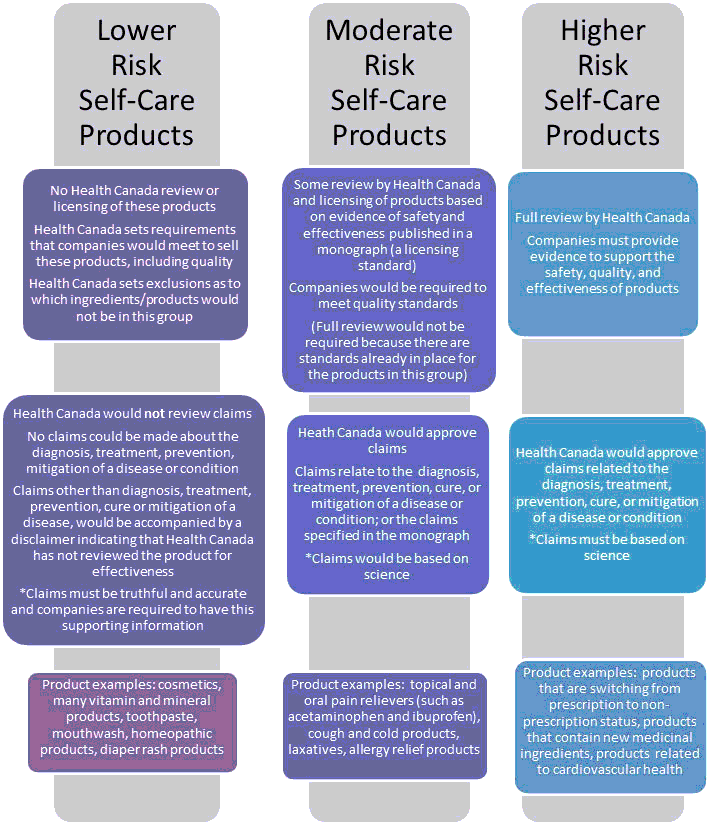
In short, if you want to sell homeopathic sugar pills, you can continue to do so. However, you will not be permitted to make any claims that your product can diagnose, treat, prevent, cure or mitigate any condition. You will also need to state on the label that Health Canada has not reviewed your product for effectiveness (which is effectively Canada’s own Quack Miranda). If you want to sell a product with a specific claim that it has a medicinal effect, then you must provide scientific evidence to substantiate that claim. The rule will apply if your product is a pharmaceutical drug, or a dietary supplement. The evidence standard and expectation will be exactly the same.
Industry spin: Health Canada is coming after your supplements!
The new requirements will not automatically force any existing product off the market. It will simply change the claims that can be made about them. If you want to claim effectiveness, then you have to substantiate your claim. The intent of the regulation is to move towards consistent standards of evidence and accuracy in label claims. This is a hard position to argue against, so the Canadian supplement industry is describing this move as one that will limit consumers. From one trade publication:
So what does the industry think about such changes? Cheryl Kos, ND, regulatory affairs research analyst at Metagenics, said that the tone and content of Health Canada’s proposal are of concern, “as is the lack of detail regarding how ingredients such as probiotics and botanicals are meant to be regulated under the proposed format and how the over 100,000 existing NHPs with NPNs and approved claims will be treated.
“The proposals appear to seek to upend a system upon which industry has spent many millions of dollars seeking to comply with, and intends to replace it with a vaguely-described new one that promises to cost more while restricting claims and product availability,” he said.
“The proposal states that consumers may believe that products situated in proximity in stores are equally effective, and seems to critique NHPs repeatedly because a lower percentage of consumers consider themselves well-informed when buying NHPs as compared to ‘other’ self-care products (OTCs and cosmetics). Neither shelf proximity nor percentage considering themselves well-informed seems a reasonable basis for determining that NHPs need to be regulated in any different way (to which they refer, in further diminution of NNHPD, as ‘modernization’),” noted Holmes.
Manufacturers are worried about losing the credibility that Health Canada’s current approval process implies. From a Canadian company that sells naturopathic shakes and homeopathy:
Health Canada’s proposal represents a marked departure from the way it has regulated NHPs for the last 12 years. The erosion of consumer confidence in NHPs may be an unfortunate outcome of the proposed new changes. The health agency’s proposed new regulation regime for NHPs is likely to result in much less choice for consumers at a time when health concerns are rising and health awareness is growing.
Health Canada would do well to consider increasing its support of NHPs in an effort to reduce rising health care costs, particularly when NHPs have a long history of traditional use, as well as being safe and effective. The government’s complete reversal on accepting as evidence a wide range of documentation to support NHP claims means health-aware consumers will be that much less informed by product labels. People make better-informed choices about their health when they have access to adequate and accurate information.
What’s more, the cost of NHPs could be driven up as manufacturers may face increased regulatory fees, additional labelling and advertising amendments, and perhaps even product reformulations.
Note how the restriction on manufacturer claims of “efficacy”, which I’ve pointed out are based on no credible scientific standards at all, are being spun to suggest that this is a negative for consumers. Consumers are absolutely are in a better position to make informed decisions about their heath when they have access to accurate information. But you can see that a company that sells homeopathy for acne, bedwetting, appetite, insect bites and more might not want to see the regulations changed if they are asked to substantiate their claims with credible scientific evidence.
Pro-science comments on the consultation
Bad Science Watch, a science advocacy group in Canada has publicly posted its own comprehensive response to the consultation [PDF]. It’s excellent and worth reading, and delves into details of the consultation I haven’t covered here. You can review their response to each question of the of the consultation. A sample:
What are your thoughts on the proposal to require scientific data to support a health claim? If you do not support, please explain why.
Health claims should of course be supported by scientific evidence. What else would a science-based regulatory body otherwise allow? An example of concern is homeopathic products that have been allowed to make specific health claims on the basis of “traditional use” despite having no scientific plausibility for efficacy and relying on generally very poorly designed (i.e., often small and/or not properly blinded) clinical research studies (Linde and Melchart, 1998, Shang et al.,2005). Traditional use should be abolished and an objective threshold of research quality and/or quantity should be included in the proposal to better define how products are able to prove efficacy prior to approval. We also argue that a requirement be set that a majority of documentation allowed to support health claims be peer-reviewed: Peer-review has been set as the international requirement for assessing validity of statements and experimental hypotheses, and as such this minimum standard should also apply to the use of scientific evidence as it applies here (Voight, 2012). Additionally, the use of scientific evidence is vital so that health providers are informed and able to discuss the safe use of health care products with patients. As an additional example, in one study health care providers were consulted about the use of echinacea-a commonly used NHP during pregnancy. Many suggested the product is safe and unlikely to be a concern, though studies do not exist to support these claims (Gallo et al., 2000).
The mandate of the Health Protection and Foods Branch can only be accomplished by use of a rigorous scientific approach.
There’s also an excellent summary of the issue in the health policy blog, Healthy Debate:
Currently, natural products are permitted to make health claims based on homeopathic textbooks, anecdotal information or the historical use to treat a condition. They’re not required to show their products have demonstrated effectiveness in controlled, scientific studies, as prescription and over-the-counter pharmaceutical drugs are.
The current regime has come under scrutiny in recent years. Last year, for example, CBC Marketplace was able to get a fake natural product licensed as an effective treatment by Health Canada. Submitting only a 1902 homeopathic reference book, the bogus children’s medication was approved to claim “effective relief from fever, pain and inflammation” on the label.
Ubaka Ogbogu, a professor of law and pharmacy at the University of Alberta, says more rigorous standards are long overdue, as there are serious dangers to taking natural health products that make unproven claims. “There’s the danger people will forgo actual treatments in favour of things that don’t work, and there’s the danger that natural health products can interact with other drugs a person may be taking,” he says. For example, the herb St. John’s Wort can interact with a variety of drugs, including blood thinners and some painkillers. (The herb can exaggerate or reduce the effects of certain pharmaceuticals). There’s also “the financial danger.” If Canadians buy an ineffective product they’ve been misled to think works, they’re wasting their money.
Canada needs you
The consultation is open for comment until October 24. Science-based comments, the more the better, are essential to add weight to support rational changes to natural health product regulations in Canada. Please consider taking a few minutes in the next several days to complete the online consultation and add your own perspectives.

