 Multiple cases of medicines killing and harming people have led, over decades, to progressive increases in the regulation and oversight of prescription and non-prescription drugs. It was the thalidomide disaster that’s primarily responsible for the current regulatory framework that is in place today, and has been successfully emulated around the world. This system ensures that products are tested before they are approved, and that products are manufactured to a consistent quality standard. With all the discussion about how the FDA’s regulatory approach for drugs might be changing, it’s worth asking how producers of products might react if regulatory standards were relaxed. History isn’t the only example we can look to. As a case study, we can look to the shelves of pharmacies and health food stores, where you’ll find hundreds of “dietary supplements” and remedies. They may look like drugs, and they may be packaged in tablets and capsules, just like medicines. But the United States, among several other countries, has enacted completely different regulations for dietary supplements. In general, they are not expected to meet the safety, quality, and effectiveness standards that are in place for medicines. In the USA, the framework is DSHEA, which removed the onus of demonstrating safety and efficacy from the manufacturer and put the requirement to demonstrate harm on the FDA – exactly the same scenario that drugs faced in the early 1900s. In Canada, the Natural Health Product regulations had a similar effect, and implemented a lowered bar for non-drug supplements.
Multiple cases of medicines killing and harming people have led, over decades, to progressive increases in the regulation and oversight of prescription and non-prescription drugs. It was the thalidomide disaster that’s primarily responsible for the current regulatory framework that is in place today, and has been successfully emulated around the world. This system ensures that products are tested before they are approved, and that products are manufactured to a consistent quality standard. With all the discussion about how the FDA’s regulatory approach for drugs might be changing, it’s worth asking how producers of products might react if regulatory standards were relaxed. History isn’t the only example we can look to. As a case study, we can look to the shelves of pharmacies and health food stores, where you’ll find hundreds of “dietary supplements” and remedies. They may look like drugs, and they may be packaged in tablets and capsules, just like medicines. But the United States, among several other countries, has enacted completely different regulations for dietary supplements. In general, they are not expected to meet the safety, quality, and effectiveness standards that are in place for medicines. In the USA, the framework is DSHEA, which removed the onus of demonstrating safety and efficacy from the manufacturer and put the requirement to demonstrate harm on the FDA – exactly the same scenario that drugs faced in the early 1900s. In Canada, the Natural Health Product regulations had a similar effect, and implemented a lowered bar for non-drug supplements.
Is there any rationale to exempt natural health products from stronger regulatory oversight? Both can have medicinal effects. Herbal remedies, if they have biological activity, are effectively unpurified, unstandardized drug products. Supporters of the industry point to the lack of harms that have been proven with dietary supplements as an argument for weaker regulation. And from one perspective, that is true. Despite their widespread use, surveillance of supplements (the limited amount that occurs) reveals generally few adverse events. It could be that many products lack meaningful pharmacologic activity — either positive or negative. An adverse event is probably less likely in placebos that have no beneficial effects. Beyond possible ineffectiveness, there are other reasons, unique to supplement use, that may contribute to the perception (and under-reporting) of harms from supplements, such as:
- Consumers believing natural products are safe may be less likely to associate adverse events with supplement use.
- The side effect profile may not be well understood, as few products are studied rigorously in clinical trials. Known side effects may not be disclosed to consumers.
- Supplements don’t require a prescription. No health professional may be involved in measuring the response to therapy, who might be able to identify adverse events.
- Side effects and harms may not be reported, as some are reluctant to share concerns with health professionals.
- Consumers may not know how to manage an adverse event, and to whom they should report an adverse event. They may be unwilling to describe health consequences to the vendor of a product, or report in a setting like a natural foods store.
- Users may have a distrust of “conventional” medicine which drove their initial use of the product. When adverse events were identified, they may be reluctant to consult “conventional” health care providers for assistance.
- Some fear of losing access to supplements, which may drive a reluctance to report adverse events.
- Supplements are commonly used for short intervals for self-limiting conditions, and long-term harms may not appear (or be detectable easily.)
With weak regulations, we really have little insight into the safety, quality, and effectiveness of many of these products.
Melatonin: Drug or supplement?
The regulation of melatonin varies around the world. In some countries, it’s regulated like a drug, and manufacturing is held to the same quality standards. In countries like Canada and the United States, it’s considered a supplement or natural health product. Melatonin is the fourth most popular natural product taken in the United States (after fish oils, glucosamine/chondroitin, and probiotics). In children, it’s second only to fish oil in terms of popularity. Sales are robust and keep growing, from $62 million in 2003 to $378 million in 2014. In the early 1900s it was observed that the pineal gland secreted a substance with physiologic effects, and the hormone itself was named in 1958. Receptors for the hormone have been identified in the hypothalamus, the pituitary, and in other organs in the body. And it’s definitely a natural substance: Melatonin is found in vertebrates, invertebrates, animals, bacteria, fungi, algae and plants. In humans and other mammals, melatonin secretion by the pineal gland follows the light cycle, being low during daylight hours, surging in the evening and peaking in the middle of the night. This secretion starts in infancy and continues through adulthood, followed by a decline as we age. It has been explored in clinical trials mainly for the prevention and treatment of sleep disorders like insomnia and jet lag.
Disappointingly and despite the fact that it is considered a drug product in some countries, the clinical evidence to support the use of melatonin is really not all that impressive. A 2014 summary of the evidence on melatonin identified six systematic reviews with seven meta-analyses, which included 9-19 randomized controlled trials with 279-1,683 patients. Studies tend to be small, of short duration, and poorly designed. Overall the data is inconsistent and relies on subjective data. When looking at all of the trials, the evidence suggests that melatonin may:
- Promote falling asleep faster by 4-11.7 minutes sooner (23 minutes faster in those with “sleep onset disorder”)
- Increase total sleep time by 8.2-18.2 minutes (four of six meta-analyses showed statistically significant increases)
- Modestly improve perceived sleep quality and efficiency (time asleep while in bed)
- In those with jet lag and shift workers, melatonin improved sleep time by 18.2 minutes but didn’t improve sleep onset or quality
In terms of short-term side effects, melatonin is well tolerated. Long-term, there’s no evidence available to determine if melatonin is safe. There’s little evidence to demonstrate that melatonin is safe in children. Moreover, it’s not even clear that when you purchase melatonin, that you’re actually getting what’s on the label.
Poor quality, contaminated supplements
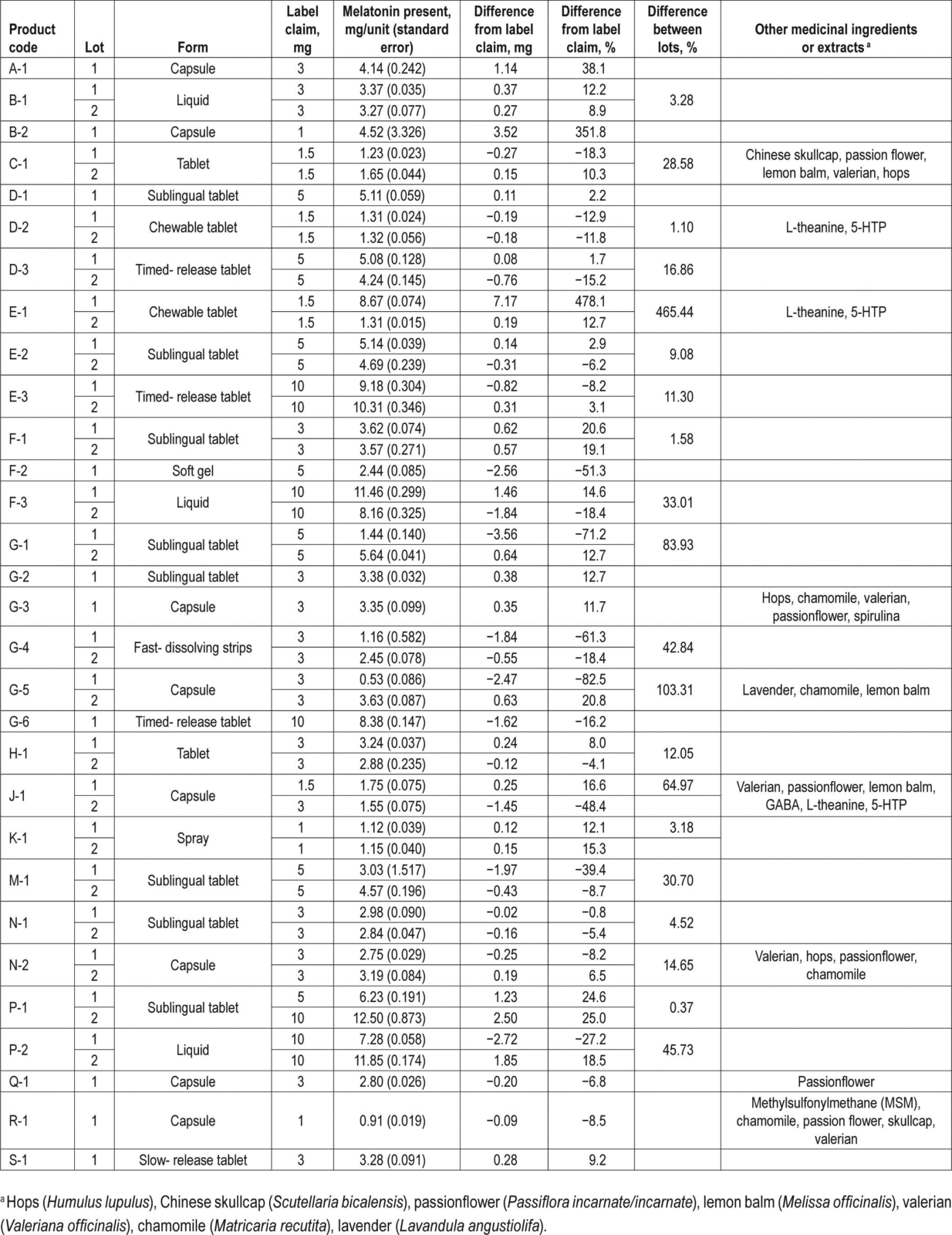
A new paper from Lauren Erland and Praveen Saxena raises concerns about the safety and quality of melatonin supplements. There was a variety of products purchased including tablets and liquids. Different lots of the same product were purchased, in order to examine variation in content lot-to-lot. The authors quantified the amount of melatonin, and also looked for the presence of serotonin. Their findings suggest that there’s little quality control in the products that were studied:
Melatonin content was found to be highly variable between samples and lots, with no pattern observed between brand, form of supplement, labelled value, or presence of other herbal extracts. The most variable sample, chewable tablet E1, showed a 478% increase from label claim containing almost 9 mg of melatonin, compared to the 1.5-mg label claim, though this was also highly variable between lots (465% difference). The supplement that showed the greatest decrease in melatonin content as compared to labelled values was the capsule G5 which contained lavender, chamomile, and lemon balm, with a decrease of 83%.
What’s on the label isn’t what’s in the bottle:
- 71% of products failed to meet label claims (per-dose content was expected to be +/- 10%).
- Actual content ranged from 83% below labelled claim to 478% above labelled claim.
- Lot-to-lot, product content varied by as much as 465%
- Undeclared serotonin was found in 8 of the products. Serotonin is a precursor of melatonin and isn’t sold as a supplement. Serotonin syndrome is a well-documented reaction with some prescription medications. The presence of undeclared serotonin could potentially lead to serious adverse reactions.
Here’s Table 1, illustrating that manufacturers of melatonin do not appear to be producing consistent, high-quality products:
Buyer beware
These finding add to other studies that have found that dietary supplements may be manufactured to poor quality standards, with little assurance that what’s on the label is actually in the bottle. Not only does it appear difficult for consumers to identify a product of high quality, there’s no assurance that product content is even consistent, lot-to-lot. What would be really interesting would be to conduct the same analysis using product purchased in countries where melatonin is regulated as a drug. In doing so, we might obtain even more insight into manufacturing quality standards for drugs versus supplements. Given the drug approval standards in place in Canada, the United States, and around the world, I’d wager that those products would be found to be far more consistent in content and quality.
Until consumers and health professionals alike have confidence that dietary supplements are of uniform and consistent quality, and are free of undeclared contaminants, it’s not possible to use them safely, or to expect that they will deliver any predictable effects.The supplement marketplace continues to serve as a case study to those that think that looser regulation actually benefits consumers, or helps ensure better health care.
Photos from flickr users planetchopstick and Michael Reuter used under a CC licence.
