A couple of months ago, one of my esteemed co-bloggers, Wally Sampson, wrote an excellent article about borderlines in research in conventional medicine. Such borderlines are particularly common in my area of expertise (cancer, which is also Dr. Sampson’s area of expertise) because there are so many cancers for which we do not as yet have reliably curative therapies. Patients faced with unresectable pancreatic cancer (as, for example, Patrick Swayze and the President of the American Medical Association have been diagnosed with) or metastatic solid cancers against which medicine generally has mostly palliative treatments, it is very tempting to take a “what have we got to lose?” attitude and pursue increasingly aggressive therapies that may actually shorten what little life a patient has left, all too often making that little bit of life more miserable than it had to be. As Dr. Sampson described in great detail, this sort of push to the borderlines and beyond led to the widespread acceptance during the 1990s of bone marrow transplantation as a treatment for advanced or inflammatory breast cancer based on uncontrolled studies that suggested a benefit. Later studies demonstrated no survival benefit (and possibly even a detriment), and that, or so it would seem, was that.
Except it wasn’t. Indeed, the other point that Dr. Sampson made was how the press covers these sorts of issues. He discussed a story that appeared in the San Francisco Chronicle about a young woman with advanced breast cancer who underwent stem cell transplantation for stage IV breast cancer at M.D. Anderson Cancer Center and was embroiled in a fight with Kaiser Permanente, her insurer, which refused to cover the treatment because it was deemed experimental and was at the time covering the cost of radiation therapy but refusing to cover the costs of extra followup scans required by the M.D. Anderson protocol. The article, not surprisingly, covered the story from the angle of the brave young cancer victim being further victimized by a greedy insurance company. And Evanthia Pappas is no doubt brave, and no one could read about her plight without rooting for her to beat the odds. The problem is that no consideration was given to just how unlikely this incredibly expensive treatment was to benefit her and whether it was even ethical to be doing such a study in which the patient bore over $200,000 of the cost for a treatment that was indeed experimental and being studied in an uncontrolled clinical trial. There are some very thorny medical, ethical, and financial issues there indeed.
Perhaps the reason Dr. Sampson’s post resonated with me was because it reminded me of a story that was extensively discussed last year, so much so that I saved the link to it. The story (Cancer Patients, Lost in a Maze of Uneven Care) appeared on the front page of the New York Times last summer. The article in question starts out by telling a truly sad story about a 35 year-old woman who, after giving birth, was diagnosed with Stage IV colon cancer as the human interest “hook” with which to represent what is described as a systemic problem with cancer care in this country:
The first doctor gave her six months to live. The second and third said chemotherapy would buy more time, but surgery would not. A fourth offered to operate.
Karen Pasqualetto had just given birth to her first child last July when doctors discovered she had colon cancer. She was only 35, and the disease had already spread to her liver. The months she had hoped to spend getting to know her new daughter were hijacked by illness, fear and a desperate quest to survive. For the past year, she and her relatives have felt lost, fending for themselves in a daunting medical landscape in which they struggle to make sense of conflicting advice as they race against time in hopes of saving her life.
“It’s patchwork, and frustrating that there’s not one person taking care of me who I can look to as my champion,” Ms. Pasqualetto said recently in a telephone interview from her home near Seattle. “I don’t feel I have a doctor who is looking out for my care. My oncologist is terrific, but he’s an oncologist. The surgeon seems terrific, but I found him through my own diligence. I have no confidence in the system.”
Ms. Pasqualetto’s heart rending case was unusual, because colon cancer is fairly uncommon in people under 50, unless they have genetic conditions that predispose them to develop the disease. The circumstances of its diagnosis, where instead of discovering the joys of being a new mother she must now battle for her life, made her case that much more tragic. However, as uncommon as it is, I have come across cases of colon cancer in patients in their 30’s often enough to know that it can happen. About a year ago, while on call I was called to see a 36 year-old man with known metastatic colon cancer who had developed a bowel obstruction. Examining him, I found large, palpable abdominal wall masses that were rock hard. This meant that there was no way I could even get into his abdomen safely, much less be likely to be able to do anything to alleviate the obstruction, and I had to tell him there was nothing that I could do. Despite the tone of this article, I would like to start by pointing out that doctors–especially surgeons–really, really hate having to tell a patient with a life-threatening or terminal illness that there is nothing they can do.
Given Ms. Pasqualetto’s shock at her diagnosis, it’s understandable that she would feel that the system doesn’t work. She did to some extent have a point in that all too often surgeons, oncologists, and radiation oncologists don’t work as much as a team as they should. Indeed that’s the very problem that comprehensive cancer centers and tumor boards (more on that below) were designed to minimize as much as possible. Certainly, this article brought out two big problem with the system, the disparities in quality in cancer care and (as always) problems with insurance coverage for such care, as will be discussed below. However, the way in which her story was presented was so laden with emotion and some assumptions about Ms. Pasqualetto’s quest for a cure that were not completely reality-based. Indeed, Ms. Pasqualetto was a rather poor example to use to discuss disparities in basic cancer care, which seemed to be the main focus of the article, and, more than that, there were also a couple of maddening things about this article that made me want to rip it up at one point the first time I read it. (More on that later.) In the meantime, here’s how Ms. Pasqualetto was diagnosed:
After giving birth by Caesarean section last July, she noticed a lump under her ribs. It was the size and shape of a banana. Doctors noticed it but did nothing. She was sent home and was told it was probably a bruise. Within a week she was back in the hospital, terribly ill — swollen with fluid, vomiting, so anemic she needed a transfusion and suffering from severe abdominal pain. Tests found colon cancer that had already spread, or metastasized, to her liver — stage 4, the final chapter of the disease.
“The doctor came in with a tear in his eye,” she recalled. ” ‘It’s bad.’ Those were his exact words. ‘You have maybe six months.'”
It should be noted that the survival for colon cancer metastatic to the liver has improved dramatically over the last couple of decades. Where it used to be around six months, median survival is now in the 14-20 month range with newer and more effective chemotherapy regimens. Indeed, if you’ll forgive a “testimonial,” my best friend’s father has colon cancer metastatic to the liver and has been doing quite well for a couple of years now, even though he does appear to have residual disease. I just saw him three weeks ago, and he still plays golf. True, he does know that sooner or later his cancer will claim him, but in the meantime he’s living what’s left of his life to the fullest, and it is advances in the therapy of metastatic colon cancer that have made that possible. My guess is that the physician who told Ms. Pasqualetto this prognosis was probably not an oncologist. Moreover, these sorts of statements to patients are exactly the sort of thing that doctors say far too often that feeds into the stereotype so frequently mentioned in “alternative medicine” testimonials of “they just sent me home to die,” even when such is not the case. (The same stereotype is at work here, just without a testimonial in which Ms. Pasqualetto turned to quackery.) Misunderstandings don’t help, either:
Surgery was not recommended because the liver tumors were too extensive. She was referred to an oncologist, who offered “palliative” chemotherapy, given strictly to ease symptoms, not to try for a cure.
“His attitude was that it wouldn’t really make a difference,” Ms. Pasqualetto said.
Here’s the misunderstanding. For stage IV colon cancer, unless the metastatic disease is confined to the liver and/or lung alone and can be completely resected surgically with no evidence of residual tumor left behind, every therapy offered will be almost by definition palliative. We can slow the progression of inoperable stage IV colorectal cancer, and we can relieve symptoms related to its progression, but, alas, we can’t cure it. Thus, when it was stated in the article that Ms. Pasqualetto’s insurance company would only pay for “palliative” care, it was not necessarily a reasonable indictment of the insurance company. As a physician, trust me when I say that I detest most health insurance companies and have pulled a few rather–shall we say?–interesting moves on occasion to get them to cover certain tests for my patients, but in this case there is just not enough information to know whether Ms. Pasqualetto’s insurance company was being too stingy in its coverage or not. Reading between the lines, my guess is that it probably only covered the FOLFOX regimen, which is pretty much standard of care for stage IV colon cancer these days, but did not cover the latest antiangiogenic drug Avastin, which when added to FOLFOX can increase median survival but is very, very expensive at approximately $100,000 a year. Regardless, even these new combination therapies are still not cures, at least not by themselves. In the absence of successful surgery that resects all the metastases, they are still palliative.
Telling a young patient that she has incurable cancer is one of the hardest things oncologists sometimes have to do. The key is not to give the patient the impression that you’ve given up or that you don’t care. It may be that her first oncologist failed at that. Or it may be that Ms. Pasqualetto just wasn’t ready to accept the hard truth yet, which would have been completely understandable but is also a common problem that oncologists need to know how to overcome. All of this combines with the very real problem described in the article, inconsistency of care among institutions and geographically, to make decisions even more difficult. If a newly diagnosed cancer patient goes for a second opinion, there is a pretty good chance that it won’t agree with the first opinion:
Even when treatment guidelines are based on solid evidence, hospitals or doctors may not stick to them. But sometimes, the science is not clear, and experts do not agree on the best course — or even on whether there is a best course.
“In cancer, there is frequently no one best doctor and no one best treatment,” said Dr. John H. Glick of the Abramson Cancer Center at the University of Pennsylvania.
When patients consult him for second opinions or to transfer their care to his center, Dr. Glick estimated that he and his colleagues concur completely with the original doctor in about 30 percent of cases. But in another 30 to 40 percent of cases, they recommend major changes in the treatment plan, like a totally different chemotherapy regimen or the addition of radiation. Sometimes his team makes a completely different diagnosis.
In about another 30 percent of cases, his team recommends minor changes in chemotherapy, or additional tests. “We interpret things differently, maybe because we have more experience,” Dr. Glick said. “We see hundreds of patients with Hodgkin’s disease. A community oncologist may see only a couple.”
What does a cancer patient do if faced with two, three, or even four different recommendations? One thing that came out of this article shocked me. Maybe it’s because I’m in the “ivory tower” of academia and can’t imagine that it wouldn’t be incredibly obvious what to do, but apparently there are a not inconsiderable number of women who undergo lumpectomies for breast cancer but do not then undergo the recommended adjuvant radiation therapy (15-25%). Apparently there are patients with colon cancer that’s spread to regional lymph nodes who don’t receive adjuvant chemotherapy. However, I’m not so isolated that I don’t know that some strange things go on out in the community. In the last eight years, I’ve had at least three patients come for a second opinion after a mastectomy was recommended for a small breast cancer, for example. Not too long ago, I was a visiting professor at a tumor board, where one of the cases presented so shocked me that I actually forgot the usual decorum a bit, in which, no matter how badly a case was handled I’m supposed to be nonjudgmental, and publicly interrogated the surgeon about what his rationale was for doing what he did. (In fairness, one of the oncologists came up to me afterward, embarrassed, and told me that they knew they had a problem with this surgeon.) Science- and evidence-based guidelines are one way to try to address this problem. In making these points, this article did a service to the public. In using Ms. Pasqualetto, whose case was anything but simple and would be prone to multiple conflicting recommendations even in a world where the disparities that this article sought to highlight didn’t exist as the exemplar of this problem, the NYT went for sympathy over substance and undermined the message.
Unfortunately, the article took it beyond that. The rest of it was couched as a quest by Ms. Pasqualetto to find a doctor willing to try to save her life, as if the doctors who refused her were being unreasonable. She found one who was willing to give her aggressive chemotherapy in hopes of shrinking her liver metastases to the point where she became operable, a perfectly reasonable approach in a patient this young. It was at this point that the article completely misrepresented a common forum where cancer therapy is discussed and decided, the tumor board:
One aspect of Karen Pasqualetto’s care has particularly troubled her. She was told that the first few months of chemotherapy had shrunk the liver tumors enough to make them operable, and surgery was scheduled for last January. She was elated, figuring that removal of the tumors was her best shot at staying alive. But in December a hospital review panel known as the tumor board refused to approve the surgery.
“I was adamantly told it was off the table, and I don’t know why,” Ms. Pasqualetto said. Even she, the feisty patient, felt powerless.
“Who is this tumor board, and do they hold the keys to my life?” she asked.
“You feel a total lack of control when you’re in a position like mine,” she said.
Her oncologist, Dr. Gold, who is chairman of the tumor board, said it was a group of doctors who met informally to review cases and decide what treatment would help a patient most. In Ms. Pasqualetto’s case, the board thought chemotherapy would accomplish more than surgery.
“Patients don’t always hear what you’re telling them,” Dr. Gold said.
While I can understand why Ms. Pasqualetto would feel that way, the NYT, by presenting the story this way, gave the impression of a monolithic, uncaring committee that doesn’t want to save her life. (Yes, this is the part of the article, where I almost ripped up the newspaper.) Basically, a tumor board is a group of doctors involved in the care of cancer who get together on a regular basis to discuss cases. Depending upon the hospital, tumor boards can range from very formal to more informal, but they are generally made up of medical oncologists, radiation oncologists, surgeons, genetic counselors, and pathologists, often with nurses and social workers also in attendance to present the patient’s side and expound on any psychosocial issues. The idea is to present and discuss cases, particularly difficult cases like Ms. Pasqualetto’s, in a multidisciplinary forum in which the committee comes to a consensus on the best course of action for the patients whose cases are presented. Indeed, it can be argued that the tumor board is a mechanism by which disparities in how cancer is treated in the community can be addressed and minimized. Also, there are multiple members of the tumor board who are involved in each case discussed; so it’s not as though the members don’t care. Tumor boards, in my experience, are great resources to help out with truly difficult cases, as Ms. Pasqualetto’s clearly was. I can fully understand why Ms. Pasqualetto was not pleased with the tumor board’s recommendation, but for the reporter to present her characterization of a tumor board stand without a better explanation of what a tumor board is and does was this article’s weakest (and most infuriating) point. It also promulgated a common misconception that patients have about tumor boards. Personally, from what I can tell from the case in this story, the tumor board’s recommendation was probably a sound recommendation. Remember, as Dr. Sampson pointed out, being more aggressive is not necessarily better. Sometimes the potential for harm cancels out the potential for benefit. Sometimes the potential for harm surpasses the potential for benefit. In Ms. Pasqueletto’s case, that certainly seemed to be true.
The final phase of the story was presented as a triumph over the system, although I’m not necessarily sure that it was. Eventually, Ms. Pasqualetto found a surgeon willing to attempt to operate, Dr. Michael Choti at Johns Hopkins, clearly a very aggressive surgeon. Now don’t get me wrong. If there’s one thing about colorectal cancer that’s metastatic to only the liver, it’s been pretty clear for a long time now that in patients whose metastases can be completely resected with negative margins there is about a 30% five year survival What’s the 5 and 10 year survival of colon cancer metastatic to the liver with only medical therapy? Very close to zero, although I will admit that the newer regimens are starting to improve that dismal number slightly. True, most patients with liver metastases aren’t candidates for surgery due to anatomy or too many metastases, but for those who are surgical candidates, the standard of care is to try to resect the metastases. The question that requires a lot of clinical judgment to decide is: Which is which? Which tumors are resectable for cure and which are not? The answer to that question is very much an issue of surgical judgment and a surgeon’s knowledge of his or her own skill. Even so, one thing that tends to drive my surgical oncology colleagues who do liver surgery absolutely batty is how often patients with potentially resectable liver metastases from colorectal primary tumors are not referred to them until it is too late and the decision has in essence been made by the disease.
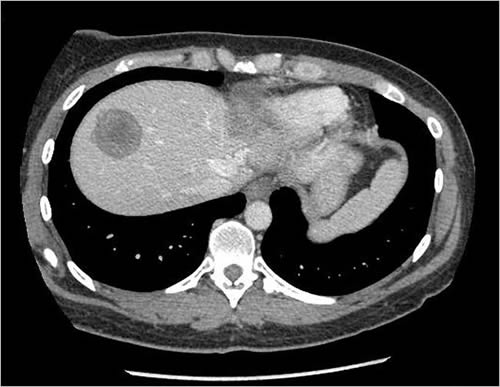
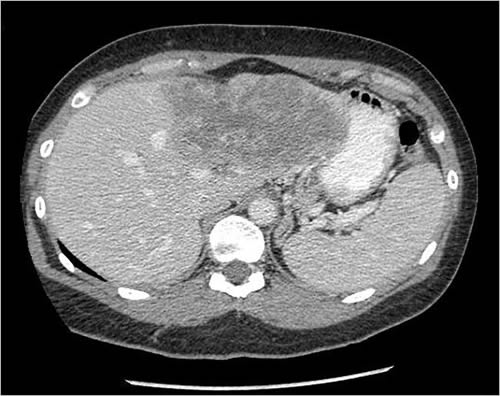
Here’s what Dr. Choti had to deal with, from two CT slices included in the article:
CT scans are viewed as “slices” seen from the patient’s feet. Consequently, the left part of the pictures above are the patient’s right side. The dark masses in the brighter area (the liver “lights up” on CT scans because of intravenous contrast) are her two tumors. One (the more defined, circular one) appears to be in the right lobe in the dome of the liver, while the other, much larger and less distinct one, in the other photo is primarily in the left lobe. This is a huge surgical challenge to remove both of these and still leave enough liver for Ms. Pasqualetto to survive until it could regenerate.) Truly, Dr. Choti must be a gifted surgeon indeed to have pulled this off with the end result being a living patient:
“They got them all,” Ms. Pasqualetto’s husband, Chris Hartinger, said shortly after her operation ended on June 21. “It turned out to be five tumors.”
Four were in her liver. The one in her colon was the size of a tangerine. Dr. Choti operated for eight hours, removing 12 to 18 inches of intestine and about 70 percent of her liver.
The day after surgery, Dr. Choti said, “I think we got away with quite a complex operation.”
The tumors were gone, but metastatic disease can be tough to beat in the long run.
Indeed, it can be, particularly given that Ms. Pasqualetto clearly had extensive disease. Dr. Choti’s tour de force operation notwithstanding, I knew at the time that she almost certainly had residual microscopic disease that would eventually blossom into mestastases again. Sadly, Googling her to try to find updates to her case in preparation for this post, I found that in December she was found to have a 7 cm recurrence in her liver, less than six months after her surgery, and that she was starting Erbitux. I do not have any more recent information, and I hope she is still doing well.
Make no mistake, though, Dr. Choti’s operation pushed the envelope, and there still isn’t really a lot of good evidence to tell us whether such massive operations truly prolong life, mainly because it’s really, really hard to do high quality randomized studies to address this question. Whether such radical resections or cytoreductive surgery, as advocated by, for example, Dr. Paul Sugarbaker, actually extend life or produce a cure in a minority of cases is something that’s argued about at the Society of Surgical Oncology meeting pretty much every year. The question is whether the apparent prolongation of life observed in patients who undergo such operations is a case of selection bias, where patients who are in good enough shape to undergo the surgery and whose tumors are indolent enough not to grow out of control before they come to surgery would be likely to do better no matter what treatment is given, or whether the surgery truly does prolong survival or even cure the disease. In other words, is the apparent survival advantage due to selecting the best patients for surgery rather than due to the surgery itself. The problem is, there’s no real practical or ethical way to do a randomized trial to answer the question; so lesser studies are what we have to base our decisions on. The answer to that question is clear in patients who have small amounts of metastatic disease that can be completely resected, in which case surgery clearly prolongs survival and even cures some patients, but it’s not so clear when it comes to large amounts of metastatic disease and disease outside of the liver. Again, such huge operations are pushing the limits of surgery and evidence-based medicine.
There is no doubt that Ms. Pasqualetto is a remarkable woman, and I certainly hope that she beats the long odds against her, especially in light of my having learned that she recurred after only a five or six month disease-free interval. However, she was a poor example for the NYT to have used as a poster child to highlight the disparities in the quality of cancer care in this nation. The reason is that she is far from a “typical” case and underwent care that is far from “typical.” Remember, the disparities addressed by the guidelines described in this article are among cancer patients who do not receive care that is overwhelmingly supported by scientific and clinical evidence as being efficacious, in other words, the standard of care. We’re talking about women with breast cancer who don’t get radiation after their lumpectomies or don’t get anti-estrogen drugs after surgery even though their tumors are estrogen-responsive. We’re talking about patients with potentially resectable pancreatic cancer, only 38% of whom are ever offered an operation to resect their cancer. We’re talking about patients with node-positive colon cancer who are not offered adjuvant chemotherapy. These are very basic things that patients should be but all too frequently are not offered. They’re Oncology 101. In contrast, Ms. Pasqualetto’s case is anything but simple. (She’s Oncology 801, if you will). Leaving aside the issues with health insurance and getting third party payers to cover her treatment, Ms. Pasqualetto’s borderline operability put her at the very borders of evidence-based medicine, where it was not at all clear what would the best treatment for her. There were several defensible treatment recommendations that could have been appropriate, ranging from a purely palliative minimalist approach (which some patients do opt for, by the way) to the über-aggressive attempt at cure that she ultimately did opt for, with many options in between. In other words, she was an outlier, not at all the “typical” patient who is not getting basic, bread and butter, standard of care treatment for common cancers.
In a “perfect” system where cancer treatment didn’t vary much from center to center, Ms. Pasqualetto might not have been able to find a surgeon who would have been willing to try to operate on her, as most guidelines would say that doing a resection of so many liver metastases that took up so much of the colon and a colon recurrence had such a low probability of saving her life and such a high probability of significant morbidity or mortality that it would, in general, not have been the recommended course of action. Admittedly in the hands of highly skilled surgeons it might be worth trying, but it would still have a pretty low chance of success. Moreover, it could be said that it’s the very variability in surgical approaches to widespread liver metastases from colorectal cancer that meant that there was a surgeon out there to be found who would be aggressive enough and skillful enough to be willing to “give it a shot” and operate on Ms. Pasqualetto. Such innovation and aggressiveness would be harder to justify in a system without such variability. That’s the biggest irony of the article.
At the risk of sounding cold and harsh, I argue that interest anecdotes designed to make a point should actually make that point, rather than be discordant with the message. It would have made far more sense for purposes of highlighting cancer care disparities to have found a woman with breast cancer who had had a lumpectomy but no radiation or who didn’t get Tamoxifen even though it was indicated and then had a recurrence of her tumor than it was to use a patient whose management was not at all clearcut and whose likelihood of dying regardless of what was done was so high. The message, namely that there are disparities in basic cancer care that need to be addressed, was a good one, but the messenger botched it by confusing basic care with complex care, much as the messenger of the article highlighed by Dr. Sampson botched the message. It is very understandable why it is so tempting to play up the emotional aspect of such stories, but doing so risks producing a distorted view of the situation.