A new year is upon us yet again, and Science-Based Medicine has been in existence for eight years now. It seems only yesterday that Steve Novella approached me to ask me to be a contributor. Our part-serious, part-facetious predictions for 2016 notwithstanding, one thing about 2016 is certain: I will almost certainly encounter some form of cancer quackery or other and deconstruct it, probably multiple forms. In any case, a topic I’ve been meaning to write about is based on a couple of studies that came out three weeks ago that illustrate why, even if a patient ultimately comes around to science-based treatment of his cancer, the delay due to seeking out unscientific treatments can have real consequences.
When a patient with breast cancer comes in to see me, not infrequently I have to reassure her that she doesn’t need to be wheeled off to the operating room tomorrow, that it’s safe to wait a while. One reason, of course, is that it takes years for a cancer to grow from a single cell to a detectable mass. The big question, of course, is: What is “a while”? Two studies published online last month attempt to answer that question. One study (Bleicher et al) comes from Fox Chase Cancer Center and examines the effect of time to surgery on breast cancer outcomes; the other (Chavez-MacGregor et al) is from the M.D. Anderson Cancer Center and examines the effect of time to chemotherapy on outcome. Both find a detrimental effect due to delays in treatment.
Prompt surgery is better
Because I’m a surgeon I’ll take a look at Bleicher et al first. This study looks at two large cancer databases, the Surveillance, Epidemiology, and End Results (SEER)-Medicare–linked database and the National Cancer Database (NCDB). The SEER-Medicare cohort included Medicare patients older than 65 years, and the NCDB cohort included patients cared for at Commission on Cancer–accredited facilities throughout the United States. Analyses performed assessed overall survival (OS) as a function of time between diagnosis and surgery and evaluated five intervals (≤30, 31-60, 61-90, 91-120, and 121-180 days). It also looked at disease-specific survival at 60 day intervals. The patient cohort included women diagnosed with invasive breast cancer that had not metastasized beyond axillary lymph nodes who were treated with surgery first. Patients with inflammatory breast cancer were excluded, which makes sense because inflammatory cancer is generally treated first with chemotherapy. The SEER-Medicare cohort included 94,544 patients 66 years or older diagnosed between 1992 and 2009, while the NCDB cohort included 115,970 patients 18 years or older diagnosed between 2003 and 2005.
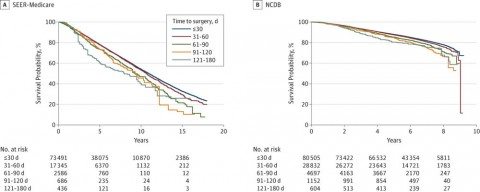
The tale is told by this graph, from the SEER-Medicare cohort and the NCDB cohort (click to embiggen):
As you can see, there is a decrease in survival with each increase in time from diagnosis to surgery, so that by the time you get to the 90 day interval it is quite striking, with a difference in long term survival larger than the improvement produced by most chemotherapy regimens. In the SEER-Medicare cohort, for each increasing interval, the hazard ratio (HR) was 1.09. Results from the NCDB cohort were similar. The added risk of death from all causes for each interval increase in time to surgery was 10% (HR, 1.10; 95% CI, 1.07-1.13; P < .001).
Now here’s the kicker. This adverse effect from delays in surgery was most marked in the very patients most likely to delay their surgery for alternative therapy, namely patients with early stage disease. In the SEER-Medicare database, for instance, the effect of delays in treatment on breast cancer-specific mortality in stage I disease resulted in a HR of 1.84 (95% CI 1.10-3.07, P = .02), while the HR for stage II and III disease was not statistically different from 1.0. In other words, a delay of greater than 120 days was associated with a nearly two-fold increased risk of dying of breast cancer. In terms of overall survival, time to surgery was correlated with a decrease in overall survival in stage I (HR, 1.13; 95% CI, 1.08-1.18; P < .001) and stage II disease (HR, 1.06; 95% CI, 1.01-1.11; P = .01), but not in stage III (HR, 1.06; 95% CI, 0.97-1.16; P = .17). In the NCDB cohort, delay in surgery was associated with decreased overall survival for stage I (HR, 1.16; 95% CI, 1.12-1.21; P < .001) and stage II disease (HR, 1.09; 95% CI, 1.05-1.13; P < .001) but not stage III (HR, 1.01; 95% CI, 0.96-1.07; P = .64). Again, patients with stage I or II disease, which can often be associated with little or no symptoms (particularly stage I, which often does not even present with a palpable mass), are exactly the ones most likely to eschew chemotherapy.
You might wonder why there is less of an effect of treatment delay in stage III disease. So did the authors:
We have found that OS declines when the TTS [time to surgery] increases, with OS affected in stage I and II but not stage III disease. The data for DSS [disease specific survival] are similar, with cancer-specific mortality data only available in the SEER-Medicare dataset, where patients with stage I cancer exhibited lower survival as TTS increased. This observation that preoperative delays affected only stage I DSS and stage I and stage II OS could be due to lower numbers of patients with higher-stage disease, but we believe that breast cancer survivability in its earliest stage is more influenced by the TTS than it is in later stages because baseline mortality is smaller relative to the effect imposed by a delay in treatment. In both cohorts, OS and DSS for stage III disease were not influenced by TTS, suggesting either partial biologic predestination of outcome or a mortality risk that overshadows any small effect of reducing delay by a matter of months. This effect may also be attenuated by patient age owing to competing mortality risks. Because of this and because final stage is only available postoperatively, we believe that efforts to minimize preoperative delay for all patients is advisable.
My thought is that the effect of a delay in time to surgery is relatively small on an absolute scale and therefore hard to detect in retrospective databases like SEER-Medicare and NCDB. Of course, it would be unethical to do a randomized, controlled clinical trial testing time to surgery; so these sorts of analyses are the best we’re going to get when it comes to answering this question. It’s also important to point out that, although the absolute value of the decrease in OS is small, it’s definitely not insignificant:
The effect of TTS on survival is a ubiquitous concern of patients with cancer and a question frequently posed in consultations with surgeons. Elimination of undue delay is desirable to both reduce anxiety and lower risk, and we believe that this study provides clinicians needed data to answer patients’ questions about TTS and its effect on outcome. While the absolute magnitude of the 5-year survival difference was small (4.6% and 3.1% for ≤30 days vs 91-120 days in SEER-Medicare and NCDB patients, respectively), this benefit is comparable to the addition of some standard therapies, such as the recent extension of tamoxifen therapy from 5 to 10 years, while not having the adverse effects or costs found with most interventions.
Exactly. As the authors point out, achieving surgery in less than 30 days is difficult and therefore 30 days might be an unrealistic goal, particularly when immediate reconstruction after mastectomy is being considered and when imaging techniques and more extensive workups can cause delays that easily stretch beyond 30 days, particularly for patients seeking multiple opinions. However, 60 days should be achievable in most cases. Either way, waiting months to undergo surgery, particularly for early stage disease, can decrease chances of survival by as much as foregoing adjuvant therapy. Speaking of adjuvant therapy, delays in chemotherapy are not good, either, as we shall see.
Timing of chemotherapy matters
The next study, Chavez-MacGregor et al, asked basically the same question, except that the authors looked at time to adjuvant chemotherapy after definitive surgery. Adjuvant chemotherapy is chemotherapy given after surgery with the intent of decreasing the chance of tumor recurrence. It is standard of care for many kinds of breast cancer. For instance, in two of the kinds of breast cancer with poorer prognosis, triple negative breast cancer [PDF] and HER2(+) breast cancer, except in the case of very small node-negative tumors, nearly every patient who is healthy enough to handle it will be recommended adjuvant chemotherapy.
Their rationale:
Most patients with breast cancer start adjuvant chemotherapy within 30 to 40 days of surgery. It is thought that chemotherapy administration delayed beyond this time can decrease the benefit provided by cytotoxic systemic therapies. Possible explanations for these effects include accelerated growth of micrometastases after resection of the primary tumor, increased tumor angiogenesis, or development of primary resistance. The optimal time of chemotherapy administration for patients with breast cancer is not precisely defined. Furthermore, it is possible that the time to chemotherapy (TTC) has a different effect according to tumor subtype, tumor stage, and tumor grade. Administration of combination systemic chemotherapy within 120 days of diagnosis in women younger than 70 years with T1cN0M0 or stage II or III hormone receptor–negative breast cancer is considered a quality metric by the Centers for Medicare & Medicaid Services. This metric will now be reported by 11 cancer hospitals as part of the Prospective Payments System-Exempt Cancer Hospital Reporting Program.
The effect of delayed TTC administration has been evaluated retrospectively with contradictory results. In a recent study, we reported that a delay of 61 or more days of adjuvant chemotherapy administration was associated with adverse outcomes among patients with stage II and III breast cancer and also among patients with triple-negative and human epidermal growth factor receptor 2 (ERBB2, formerly HER2 or HER2/neu)-positive tumors. Our findings suggest that among these specific patient subgroups, every effort should be made to avoid delayed adjuvant chemotherapy initiation.
This study didn’t use the SEER database or NCDB. Instead it examined a total of 24,843 patients from the California Cancer Registry diagnosed with stage I to III breast cancer between January 1, 2005 and December 31, 2010 treated with adjuvant chemotherapy. Time to chemotherapy was defined as the number of days between the last surgery for breast cancer and the first dose of chemotherapy, and delayed time to chemotherapy was defined as 91 or more days. Overall, the authors found no evidence of adverse effects when patients started chemotherapy between 31-60 or 61-90 days after their surgery as compared to patients who started their chemotherapy in 30 days. However, for patients who started their chemotherapy 91+ days after their surgery the results weren’t so good. These patients experienced worse overall survival (hazard ratio [HR], 1.34; 95% CI, 1.15-1.57) and worse breast cancer–specific survival (HR, 1.27; 95% CI, 1.05-1.53). The authors then did a subgroup analysis examining different subtypes of cancer to subtype, longer time-to-chemotherapy was associated with worse OS in the subgroups one would predict. Specifically patients with triple-negative breast cancer had worse overall survival (HR, 1.53; 95%CI, 1.17-2.00) and worse breast cancer–specific survival (HR, 1.53; 95%CI 1.17-2.07). This finding intuitively makes sense because it is patients whose tumors are estrogen receptor-negative for whom there is the greatest benefit due to adjuvant chemotherapy.
Not surprisingly, the authors found a correlation between prolonged time to chemotherapy and Hispanic ethnicity, non-Hispanic black race, lower socioeconomic status, and nonprivate insurance. This is similar to what Bleicher et al found with respect to time-to-surgery, namely that the proportion of patients with black race or Hispanic ethnicity increased with each interval delay. This is by no means a new finding; disparities in health care of this sort have been documented in many previous studies. Indeed, these sorts of disparities are likely one reason (of many) why minorities and people of lower socioeconomic status experience worse outcomes in many cancers. Indeed, there are a lot of potential confounders, many of which couldn’t be accounted for in either study, as Chavez-MacGregor et al note:
Our study is limited by its retrospective nature. However, that we are aware of, this study is the largest published cohort of patients with breast cancer of known breast cancer subtype treated with contemporary regimens. We acknowledge that in clinical practice a number of factors determine the optimal TTC, and that in many cases, this time frame is determined by comorbidities or complications associated with surgery. Unfortunately, data concerning comorbidities and complications with surgery are not available in the CCR data database, and we cannot exclude that the factors associated with delays in chemotherapy administration are not also related to worse outcomes. However, the fact that we observed consistent results in our OS and BCSS [breast cancer specific survival] risk estimates makes this scenario unlikely. In addition, we acknowledge that the potential determinants of chemotherapy initiation include the recommendation of the medical oncologist and the entire multidisciplinary team. Additionally, from the patient-centered care perspective, a patient’s preferences are likely to play a role, which we were unable to take into account.
Similar comments apply to the time-to-surgery study, based on the strengths and weaknesses of each database used. But, again, it’s unethical to do a randomized trial studying a question like this.
The bottom line: Timely treatment is better than delay
I realize that these two studies are about as close to “Well, duh!” studies as there are. Of course, delaying surgery for breast cancer is not a good thing. Of course, delaying chemotherapy when it’s indicated is also not a good thing. These are results that are not unexpected. However, these studies are still very important because they give us estimates of how much of a delay is safe and at what point delaying care starts to have a measurable impact on patient outcomes. Putting the results of these studies together suggests that it’s best to do surgery within about 60 days in patients not needing chemotherapy first, and that for patients with disease lacking the estrogen and progesterone receptor it’s best to start chemotherapy within 90 days of surgery.
We can thus reassure anxious patients who want their surgery tomorrow while at the same time tell patients balking at surgery or chemotherapy how long they can safely wait before the delay starts adversely affecting their chances of survival. Unfortunately, in my practice, due to the socioeconomic status of a lot of patients, by the time some of my patient see me it’s already been more than 30 days since their biopsy and diagnosis.
This sort of analysis is also yet another bit of data demonstrating that conventional treatments work. After all, if a conventional treatment didn’t work, it wouldn’t matter how long you waited to administer it. For instance, if you treated a woman with breast cancer with homeopathy right away, the results would be the same if you waited 120 days. I’ve discussed examples of patients who paid a steep price for their delaying effective treatments for their cancers, beginning with breast cancer patients over eleven years ago and a man I encountered as a resident with rectal cancer who had turned himself orange with megadoses of carrots trying to treat his disease. We also know that the use of alternative medicine as a primary treatment in breast cancer is associated with recurrence and death. Using alternative therapy for breast cancer is a good way to die when you don’t have to or to die sooner than you would otherwise. Refusing surgery also results in death.
Fortunately, however, relatively few patients rely only on alternative medicine. I mention them mainly because the delays in treatment leading to unnecessary death from such treatments has been a major theme of my blogging over the years. However, where this study is most helpful is in providing patients with evidence-based recommendations regarding how urgently they need to proceed with treatment, calming those who think they’re going to die if they don’t get their surgery or chemotherapy tomorrow, and trying to persuade those who are indecisive or too slow to act. They will also help those of us in quality improvement determine whether time to treatment should be a metric we measure and, if so, what standards we should set. Prompt treatment is better, but it’s tricky to define what exactly constitutes “prompt.” These studies help.