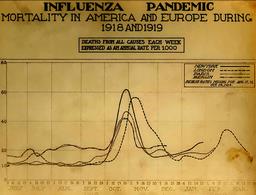
The worst flu season ever. And it peaked early.
I was asked this question by one of my infection control practitioners. We started offering the flu vaccine the first week of October and some have delayed getting the shot for fear that immunity will not last the season. She pointed me to this NPR article, “Yes, It Is Possible To Get Your Flu Shot Too Soon,” as driving the fear of too little too soon.
So when should you get the flu shot?
Short answer – I don’t know.
There are multiple variables that make it a difficult question to answer. When does flu season start? When does it end? Influenza has immunologic variation from year to year and different strains circulate each year. How good is the match between the circulating strains of influenza and the vaccine? How good is the immunologic response of the host?
So many questions. By answering these question maybe we can come up with an estimated optimal time to receive the flu vaccine.
When is flu season?
When is the flu season? It usually starts in the mid-fall, rapidly rises in the winter, then just as rapidly declines.
flu activity most often peaked in February (14 seasons), followed by December (7 seasons), March (6 seasons), and January (5 seasons).
So you would want to have antibody from December to March for protection during the worst of the flu season.
Except for H1N1, which had the audacity to begin in the spring. And the great pandemic of 1918 that peaked in October.
It takes about two weeks to develop an antibody response after the vaccine, so if you go back six months from the latest historical peak of the flu season, that would be more or less October.
There are sporadic cases of flu during October and November as influenza stutters along but never really gets a hold. Why is that? Is it herd immunity? Waiting for declining vitamin D levels? Not enough indoor crowding? I don’t know, but I suspect a little bit of each.
Part of the problem with the flu vaccine is that it offers less than perfect protection combined with less than perfect uptake. With 60% of children and 40% of adults vaccinated last year, and a 60% effectiveness, the US did not have anywhere near the vaccination rates that would be expected to stop the spread of diseases.
I would wonder if maximizing vaccination early would have an effect on the spread of influenza later in the year, minimizing the effect on the population as opposed to the individual. I still want a year where the vaccine is only offered west of the Mississippi (I live in Oregon) and mandated for everyone under pain of having to move East.
Me versus the world
But I am writing in the US, societal benefits be damned, how will the timing of the vaccine help me? Me me me.
One answer may be to know how long neutralizing antibodies last. As always, it depends on the population studied.
HIV-infected women had lower seroconversion rates (ranging from 63%-92% vs 36%-40%), lower antibody titers through postpartum week 24, and overlapping antibody half-lives (ranging from 106-121 vs 87-153 days)
If you are elderly, the antibody response to the vaccine may last for a year, and is better if there was preexisting antibody:
These findings demonstrated that the elderly living in the community developed adequate antibody responses with sustainable titers throughout the 12-month study period after influenza vaccine immunization. Moreover, the presence of pre-existing antibody at a titer ≥40 prior to vaccination strongly affected the antibody response to influenza vaccination.
And the increased dose vaccine gives a higher antibody peak and a slower decline in antibody levels.
For H1N1? Protection lasts about 2 years, although there was some antibody from the 1976 Swine flu vaccine still active against H1N1.
And there are more of the same.
So how long do antibodies from the flu vaccine last? It depends on the strains in the vaccine and the host vaccinated and the type of vaccine, but most of the time for most of the strains in most of the hosts, the antibodies mostly last the season.
Mostly.
But it is well known that antibody levels do not necessarily correlate with protection against disease. How about more real world results? In the 2011 season in Spain,
The VE (vaccine effectiveness) was 61% (95% CI: 5 to 84) in the first 100 days after vaccination, 42% (95% CI: -39 to 75) between 100 and 119 days, and zero thereafter. This decline mainly affected people aged 65 or over. These results suggest a low preventive effect of the 2011/12 seasonal influenza vaccine, and a decline in VE with time since vaccination.
So good for three months. But that was for a year with a poor match between the circulating strains and the vaccine.
For the elderly against H2N2 in 2011 in a late season and with a limited match between the vaccine and circulating strains? Notice the qualifiers?
The estimated influenza VE was 52% (95% CI, -3 to 78), 40% (95% CI, -40 to 74) and 22% (95% CI, -135 to 74) at 3.5 months, 3.5-4 months, and >4 months, respectively, since vaccination. A decrease in VE with time since vaccination was only observed in individuals aged ≥ 65 years.
And this was before the high dose vaccine.
We found an overall adjusted VE that provided significant and fairly consistent protection ranging from 54% to 67% during 0-180 days post vaccination.
So six months. But this was in a young population over four flu seasons (2010-2013).
But why does the efficacy of the vaccine decline in some populations and not others? It could be declining immunity, but it could also be due to drift of the virus during the season. The strains at the start of the season are immunologically different from the strains at the end of the season, so the vaccine would prevent disease at the start season would not prevent disease at the end of the season due to increase in mismatch, in which case delaying the vaccine would set you up for an early case and not help prevent a case later in the flu season. In other words, waiting could make you more vulnerable early in the season and still not protect you later.
Adjusted IVE was 38% (95%CI: -8 to 65) in the early influenza season (up to week 6 of 2012) and -1% (95% CI: -60 to 37) in the late phase. The results suggested a low adjusted IVE in 2011/12. The lower IVE in the late season could be due to virus changes through the season or waning immunity.
So, when should I you we get the vaccine?
So looking at the start of the season, the peak of the season, the type of vaccine (usual, adjuvanted, increased dose), the circulating strains, the host (young, old, transplant, HIV+ etc), antibody kinetics, and the clinical trials, when should you get the flu vaccine?
Got me.
But playing the odds, it looks to average out to about October to mid-November at the latest. That will provide protection at the usual start of season and during the usual peak. Which is when I am going to get mine. August and September could be too soon, December too late.
As the CDC suggests:
Optimally, vaccination should occur before onset of influenza activity in the community. Health care providers should offer vaccination by the end of October, if possible. Vaccination should continue to be offered as long as influenza viruses are circulating. While seasonal influenza outbreaks can happen as early as October, most of the time influenza activity peaks between December and March, although activity can last as late as May. Since it takes about two weeks after vaccination for antibodies to develop in the body that protect against influenza virus infection, it is best that people get vaccinated so they are protected before influenza begins spreading in their community.
I like it when the CDC agrees with me.
Addendum
After the final draft, this came across the web:
“Modest Waning of Influenza Vaccine Efficacy and Antibody Titers During the 2007–2008 Influenza“:
Time-varying vaccine efficacy (VE[t]) was examined in healthy adult participants (age range, 18–49 years) in a placebo-controlled trial of inactivated influenza vaccine (IIV) and live-attenuated influenza vaccine (LAIV) performed during the 2007–2008 influenza season…Overall efficacy was 70% (95% confidence interval [CI], 50%–82%) for IIV and 38% (95% CI, 5%–59%) for LAIV. Statistically significant waning was detected for IIV (P = .03) but not LAIV (P = .37); however, IIV remained significantly efficacious until data became sparse at the end of the season. Similarly, antibody titers against influenza virus hemagglutinin and neuraminidase significantly decreased over the season among IIV recipients… Conclusions. Both vaccines were efficacious but LAIV less so. IIV efficacy decreased slowly over time, but the vaccine remained significantly efficacious for the majority of the season.
