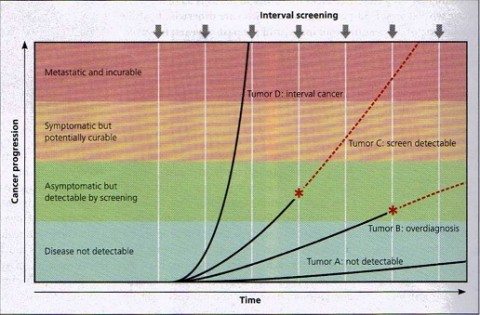
Screen detection and tumor growth rates. Cancers have different growth rates, which determine their potential to be detected by screening. Tumor A remains microscopic and undetectable by current technology (although more sensitive tests in the future might render it detectable). Tumor B eventually becomes detectable by screening (*), but its growth rate is so slow that it will not cause symptoms during the life of the individual; its detection will result in overdiagnosis. Tumor C is capable of metastasizing, but it grows slowly enough that it can be detected by screening (*); for some, this early detection will result in survival. Tumor D grows very quickly and therefore is usually not detected by screening. This will present as an interval cancer (i.e. detected clinically in the interval between screening examinations) and has a particularly poor prognosis. Note that of the four tumor types, only Tumor C has the potential to benefit from screening. Red dashed lines represent the natural history of a tumor in the absence of detection by screening. (Figure 1 from Gates, 2014).
A new stool DNA test was recently approved by the FDA for colon cancer screening. My first reaction was “Yay! I hope it’s good enough to replace all those unpleasant, expensive screening colonoscopies.” But of course, things are never that simple. I wanted to explain the new test for our readers; but before I could start writing, some other issues in cancer screening barged in and demanded to be included. They exemplify the dilemmas we face with every screening test. We have covered these issues before, but mainly in reference to mammography and prostate (PSA) screening. My article morphed into a CLT sandwich: colon, lung, and thyroid cancer screening.
The current issue of American Family Physician has a great article on cancer screening. It uses lucid graphics to illustrate lead time bias, length time bias, and overdiagnosis bias, as well the effect of varying tumor growth rates on screening success rates, all concepts that have been covered by Dr. Gorski here. Briefly, screening may do more harm than good if:
- It detects cancerous cells that never would have developed into invasive cancers or harmed the patient in any way;
- Early diagnosis and treatment decrease quality of life without reducing death rates; or
- The test falsely indicates cancer in patients who don’t have it or fails to indicate cancer in some who do.
Colon cancer
Current recommendations advise screening after age 50 by any of three methods: colonoscopy every 10 years, flexible sigmoidoscopy every 5 years, or fecal occult blood (FOBT) testing every year. Sigmoidoscopy and FOBT screening have been shown to reduce the number of deaths from colorectal cancer, but colonoscopy has not; studies are in progress. There is as yet no good evidence that any colon cancer screening test reduces overall mortality. Colonoscopy is considered the gold standard because polyps and cancers can be directly visualized and biopsied, but as yet there is no good evidence from controlled studies to confirm our reasonable assumption that it ought to be the best screening test to reduce deaths from colon cancer. It is generally considered the preferred screening option; but it is invasive, requires an unpleasant bowel prep, is unacceptable to many patients, is expensive, requires sedation, carries risks, and consumes a lot of specialist time and resources.
Colonoscopy identifies cancers directly; other tests use indirect methods. Blood in the stool can be a sign of colon cancer, and fecal occult blood testing (FOBT) uses guaiac to detect blood in the stool that isn’t obvious to the naked eye. It requires three stool samples and avoidance of foods that could cause a false positive test. Fecal immunochemical testing (FIT or iFOBT) requires only a single sample with no dietary restrictions, and it is less likely than guaiac testing to confuse bleeding from the upper digestive tract with bleeding from the colon.
The new DNA test (Cologuard) also requires only a single specimen and no dietary restrictions. It looks for DNA markers associated with colorectal cancers as well as for blood. It detects more polyps and more cancers than previous fecal tests, but has more false positive results.
According to The Medical Letter, the new DNA stool test “detected 92% of cases of colorectal cancer in asymptomatic average-risk persons, but it detected less than half of advanced precancerous lesions and produced a substantial number of false-positive results.” The appropriate interval for screening has not been established; Medicare has approved reimbursement for testing every 3 years with a preliminary pricing decision at $502. A positive screening test must be followed by colonoscopy. So this test is promising but it is no panacea, and questions remain. We’ll have to wait for controlled studies to tell us whether it can replace colonoscopy screening or reduce all-cause mortality. Until we have that data, it is a reasonable option for patients who refuse colonoscopy.
Lung cancer
Lung cancer is responsible for 27% of all cancer deaths in the US, and 33% of deaths in smokers. We used to do annual chest x-rays, but they missed small cancers and the larger ones they detected were often untreatable. We stopped doing them once we realized they didn’t improve survival. With computed tomography (CT) there is a better chance to detect small cancers that have not yet metastasized. If we did annual CTs on everyone, we would get way too many false positives and expose people to radiation for no benefit, so screening is directed at high-risk individuals. Since 1999 several studies have evaluated low-dose CT to screen smokers for lung cancer. Screening reduces all-cause mortality by 6.7%. To detect one case of cancer, 320 smokers have to be screened for 5 years.
There are some problems:
- Patients at lower risk are unlikely to benefit.
- Radiation is involved; and even at low dose, the cumulative exposure over a lifetime of screening could be harmful.
- It leads to over-diagnosis and unnecessary invasive surgeries.
- It’s expensive.
- It’s a technological Band-Aid for a lifestyle problem that would be better addressed by getting patients to stop smoking.
The United States Preventive Services Task Force currently recommends annual screening for lung cancer with low-dose CT in adults aged 55 to 80 years who have a 30 pack-year smoking history and currently smoke or have quit within the past 15 years. Screening should stop when the person has not smoked for 15 years or develops a health problem that substantially limits life expectancy or the ability or willingness to have curative lung surgery. The American Academy of Family Physicians adds that it should only be done in conjunction with smoking cessation interventions.
Thyroid cancer
Korea is in the midst of an apparent thyroid cancer epidemic: between 1993 and 2011, the incidence rose by a factor of 15. More than 40,000 Koreans were diagnosed with thyroid cancer in 2011. The rate of diagnosis has skyrocketed, but the death rate has not changed; the number of deaths from thyroid cancer has remained stable, between 300 and 400 deaths a year. This isn’t a true epidemic; it’s a consequence of over-diagnosis from injudicious screening.
Korea has national health insurance. In 1999 it implemented a free national screening program for cancers of the breast, cervix, colon, stomach, and liver. Ultrasound screening for thyroid cancer was offered as an add-on for a small copayment. Many people who opted for ultrasound screening were found to have small cancers. We have long known that at least one third of adults have small thyroid cancers; most of these never produce symptoms and are only found at autopsy after people die of something else. Guidelines recommend against surgery for tumors smaller than 0.5 cm, but the guidelines are frequently disregarded. Most patients are treated: 2/3 with radical thyroidectomy, 1/3 with subtotal thyroidectomy. Most of them require lifelong thyroid replacement therapy, 11% develop hypothyroidism, and there are surgical complications including a 2% risk of vocal cord paralysis and even a small risk of death.
The incidence of thyroid cancer diagnoses has also more than doubled in the US and several other countries. Ultrasound has a role in diagnosis, but indiscriminate ultrasound screening for thyroid cancer clearly does more harm than good, and can’t be recommended.
Conclusion
You might think we should always screen everyone for all kinds of cancer because early detection saves lives. We shouldn’t always, because sometimes it doesn’t. This is a hard message to get across to the public and even to some doctors.
Technological advances will continue to improve screening tests; but we will always face difficult decisions about who to screen and when.
Addendum
This article originally said Cologuard does not test for blood, but a company representative contacted me with a clarification: it does test for blood along with DNA markers. I also said Medicare was considering coverage, but Medicare has now made a decision. I have updated the text accordingly.
