Should supplement manufacturers be required to back up their efficacy claims with credible evidence?
Back in September, I wrote about the consultation undertaken by Health Canada (Canada’s version of the FDA) to overhaul the way it regulates dietary supplements (they call them, somewhat misleadingly, “natural health products”.) Health Canada is proposing a move towards a risk-based licensing framework that will apply to all types of self-care products. This will eliminate the special consideration currently applied to natural health products which undergo only a cursory review, yet are permitted to make scientifically-unsubstantiated claims on their labels. Health Canada’s newly-proposed regulatory approach will more tightly link specific efficacy claims with the requirement to provide scientific evidence to justify these statements. Low-risk products, like the pseudoscience of homeopathy, wouldn’t be permitted to have scientific claims at all In fact, they wouldn’t even be reviewed by Health Canada, eliminating the perception among Canadians that Health Canada’s approval is a signal that a natural health product is both safe and effective.
The proposal, which I noted wasn’t perfect, is much more aligned with science than the current licensing standard, which are, for lack of a better word, myth-based. Health Canada will currently approve virtually any product, with seemingly any claim, and for which zero credible evidence needs to be provided. While health regulations should exist to protect consumers, Health Canada has consistently given manufacturers the upper hand, prioritizing a company’s desire to sell a product over a consumer’s right to a properly regulated marketplace with safe, effective, and accurately-labelled products. CBC Marketplace proved this when it obtained approval for a fake homeopathic remedy, substantiated by a few photocopied pages from a homeopathy textbook. After that embarrassment, Health Canada tweaked its regulations, but not enough to really address the root cause of the problem, which continues to be a regulatory double standard. Being based in evidence, I predicted that the supplement industry wouldn’t be happy with the new proposal. And judging by the feedback to Health Canada’s consultation, it looks like an effective campaign was mobilized. Because if these results truly represent the views of Canadian consumers and health professionals, neither group seems to care about the accuracy of health claims made by supplement manufacturers – they actually oppose proposal to make them factual. Do consumers and health professionals really prefer ignorance over facts?
The consultation
The consultation was launched in September and closed in October 2016, allowing anyone to contribute to discussions about how different types of self-care products (including cosmetics, dietary supplements, and non-prescription drugs) are regulated in Canada. The key components of what Health Canada is proposing are as follows:
- Products with similar risk profiles would be treated (from a regulatory perspective) in the same way.
- This will ensure consistent and easier-to-understand rules for products whether they are supplements or drug products.
- Health Canada would review claims based on a new definition of a health claim.
- Companies would be required to submit scientific evidence to support claims.
- A risk-based approach would be implemented which would allow Health Canada to withdraw unsafe products.
Participants were invited to review a policy document before commenting. However it appears large number simply pasted in identical responses, in some cases seemingly not even responding to the facts of the proposal itself.
The response
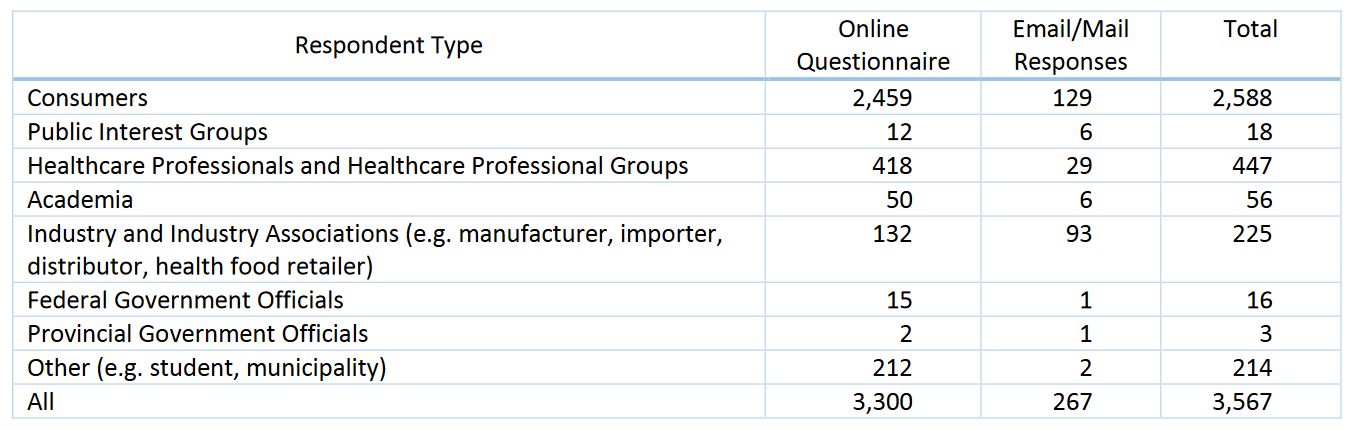
The full report on the responses is available on Health Canada’s website. In total there were 3,567 responses of which 2,588 self-identified as consumers:
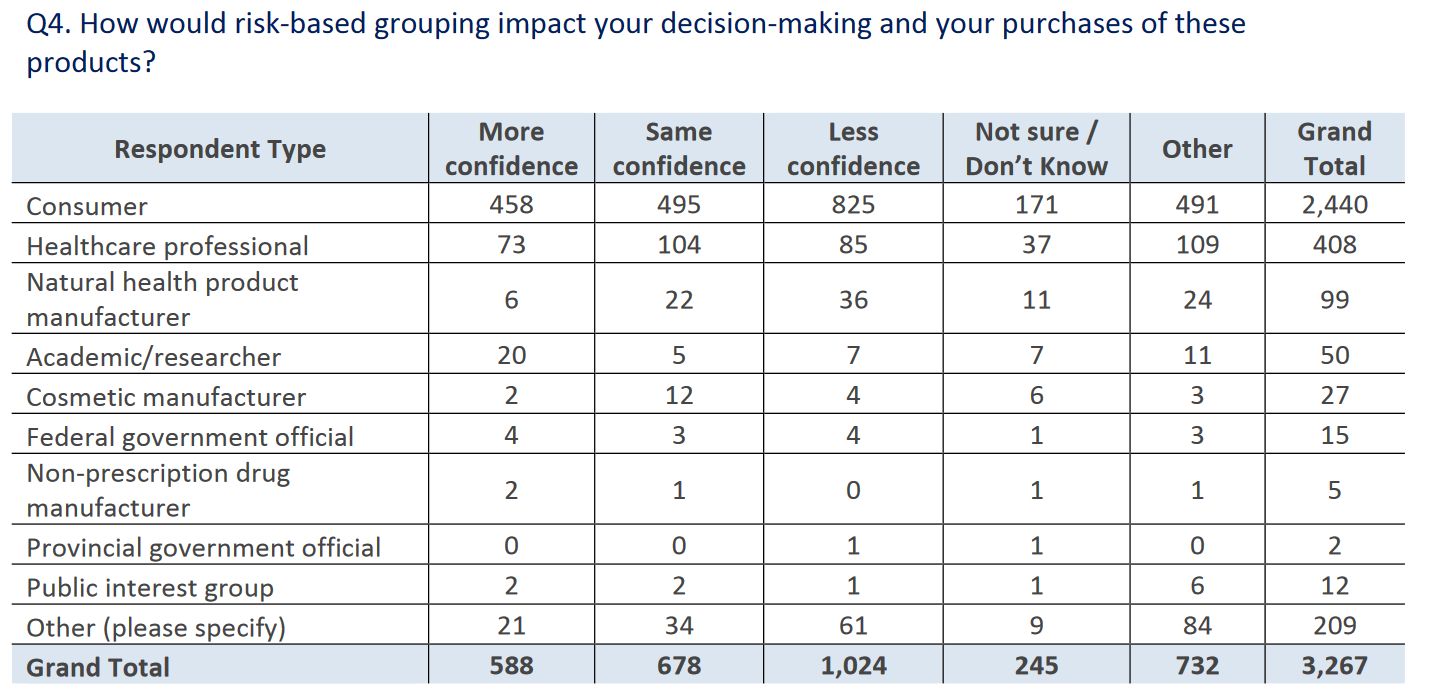
Health Canada noted that responses were “polarized” and “diverse”. There was mixed- to-negative support for grouping and regulating products based on risk (versus the status quo, which is a lowered bar for natural health products). Most respondents suggested that this approach would give them less, not more, confidence in decision-making:
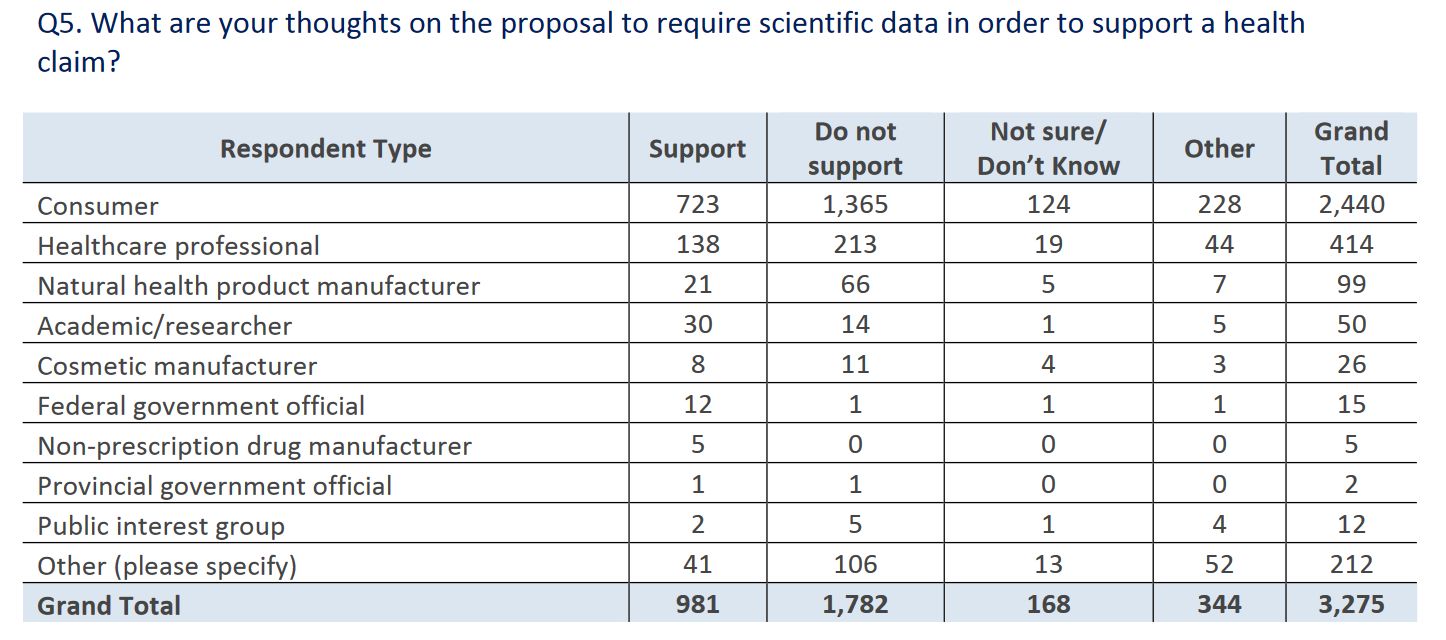
It’s the next question where I wondered if the report was written by George Orwell. When it comes to scientific data, consumers and health professionals apparently don’t want it forming the basis for health claims:
The consultation notes that there were fears of this requirement affecting the “affordability, availability and diversity” of products sold, which suggests that many didn’t understand the proposal itself, which was related to claims, not availability.
I can’t think of another consumer good or any marketplace of any kind where consumers would actively support allowing manufacturers to make unsubstantiated claims. Holding manufacturers to account harms no-one except the manufacturer’s financial interest. Consumers would benefit from access to only substantiated claims, rather than the current standard of unsubstantiated claims. Yet overwhelmingly, this aspect of the consultation was not favored by respondents.
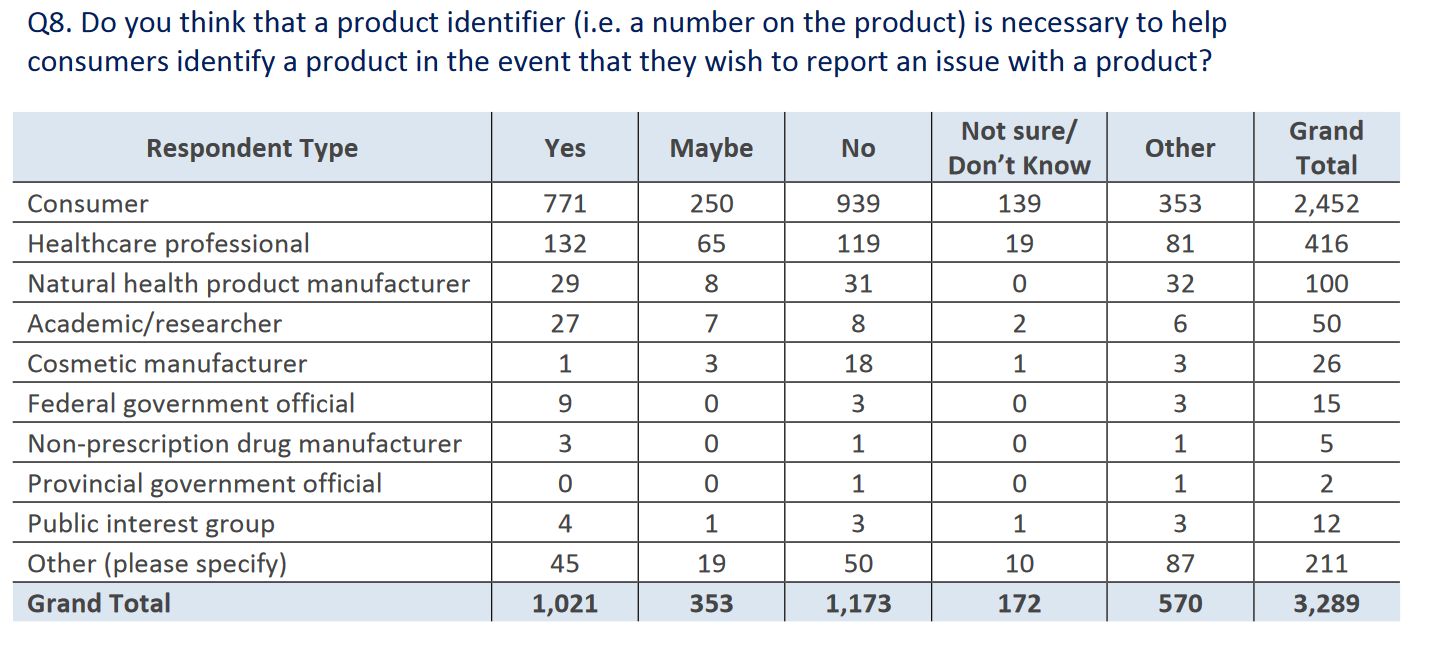
The puzzling responses continued in question 8, which asked if a unique product identifier should be placed on the package to support identifying problematic products, or allowing easy identification in the case of a recall. Again, there was more opposition than support for this proposal. Why this would be opposed at all is a bit baffling to me, as it harms no-one and only has the potential to benefit consumers. At least healthcare professionals were slightly in favour:
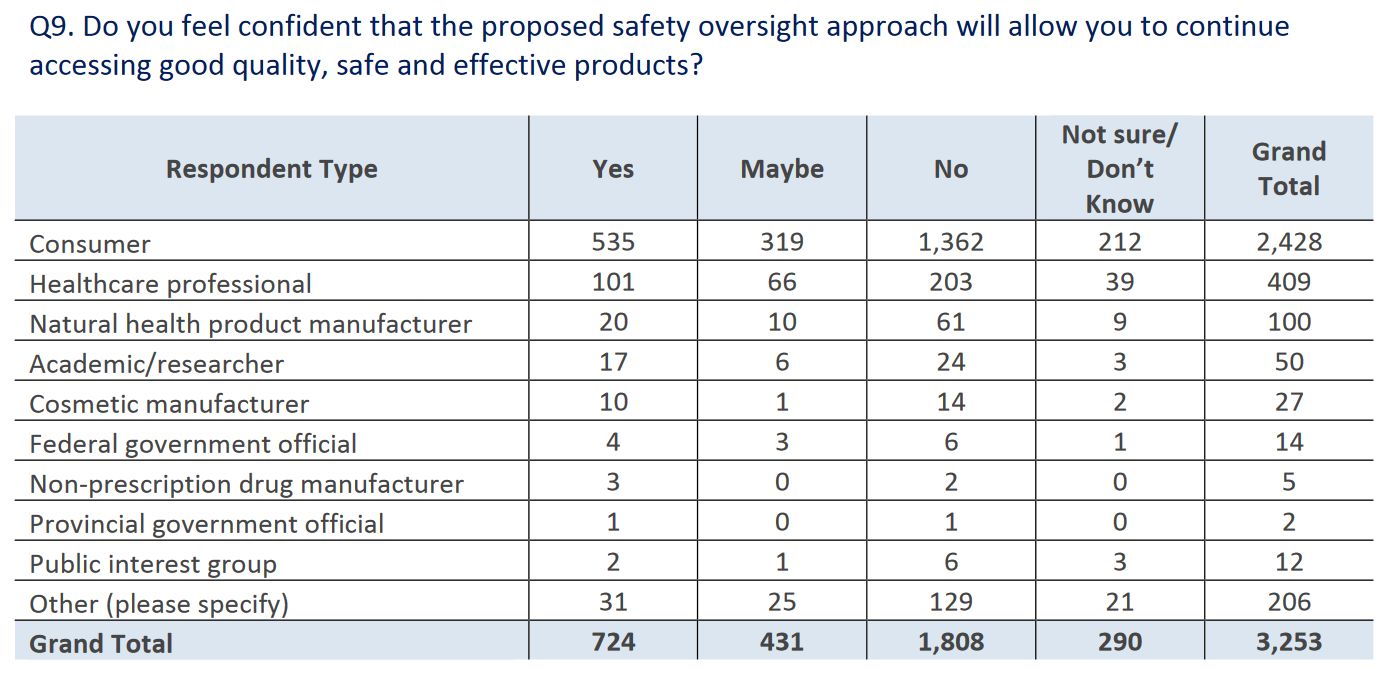
Overall, respondents were not supportive of the proposed strengthened regulations, and seemed concerned that it will limit access to products:
Industry mobilization campaigns
The report comments on two mobilization campaigns:
Health Canada is aware of two public mobilization campaigns which were undertaken during the online consultation period.
First, a petition was developed and publicized that urged the Prime Minister and Cabinet Ministers to protect the interests of local producers of medicinal herbs against limitations that might be imposed by a regulatory requirement for proof of efficacy. This petition received 1,514 signatures.
A second campaign, which implied that Health Canada is proposing to regulate NHPs as prescription drugs, encouraged members and supporters to complete the consultation questionnaire using elements from a template of answers which was created and published online. The group generally opposed all aspects of the proposed modernization and encouraged members and supporters who shared this view to respond to the consultation and use its template when doing so. The template provided clearly affected the overall tone and content of responses to the consultation questionnaire. In fact, of the 3,300 questionnaires completed by participants, 493 (or 15%) responded to six or seven of the seven closed-ended consultation questions exactly as the advocating group had proposed. An analysis of the open-ended responses provided by these participants also showed a close match to the text content suggested by the advocating group, which suggests that respondents may have taken their lead from the campaign.
The impact of this campaign on the overall results of the consultation cannot be discounted. The results of the campaign are important overall context for understanding the consultation results, as these respondents are typically exclusively interested in the future of NHPs under the proposed modernization rather than in the future of all self-care products. It is important to note that presently, when looking at cosmetics, NHPs and NPDs, the most modern set of regulations and the most risk-based regulatory structure is the one in place for NHPs. As such, it is possible that the potential benefits the proposed approach would bring are less evident for NHPs specifically when compared to cosmetics and NPDs.
What happens next?
As Carly Weeks noted in The Globe and Mail, Health Canada, to its credit, seems undeterred:
On Friday, the department released a report on the consultation process that revealed strong opposition to changing the way natural health products are regulated. Much of the opposition came from members of the public responding to an advocacy campaign led by members of Canada’s natural health products industry, which warns that the new system would limit the availability of vitamins, supplements and similar products.
And:
While the government is considering all the responses, it remains committed to updating the regulatory system, Ms. Bombardier said. According to the report, some of the public opposition stems from misconceptions promoted by the natural health industry, such as the inaccurate assertion that Health Canada wants to regulate natural health products the same way it regulates prescription drugs.
For instance, the Canadian Health Food Association, which led a campaign against the proposed changes, suggests on its website that the new system will reduce oversight on some natural health products and increase it on others, requiring “a level of evidence similar to what is needed for drugs.”
Health Canada has announced another round of in-person and online consultations, starting this month. If the feedback to the initial survey is any sign, we should expect the supplement industry to continue to mobilize and push hard to derail any attempts to strengthen regulations. Ultimately, Health Canada is accountable to all Canadians, not just supplement manufacturers. Health Canada’s new proposals have the potential to repair much of the damage that’s resulted from a lax approach to regulation. Restricting manufacturers from making claims that aren’t backed by good science isn’t a question that should even need to be asked – nor should it be subject to polling. It’s an essential component to fairness in any health market that prioritizes public health over manufacturer interests.
Photo via flickr user Shannon Kringen used under a CC licence.
