[Editor’s note: Sorry that this published about 12 hours later than normal. Somehow, I had the draft all done yesterday but forgot to set it to publish at the usual 3 AM time. Better late than never, hopefully.]
Back in February, I wrote about an incident demonstrating how antivaccine activists weaponize vaccine safety studies to falsely portray vaccines as more dangerous than the disease. The example that I used was a study published by a large multinational research group in the journal Vaccine using the Global Vaccine Data Network (GVDN) to examine thirteen medical conditions that the group considered “adverse events of special interest” (AESI) potentially associated with COVID-19 vaccination. The study involved examining the records of more than 99 million vaccinated individuals in eight countries, with the intention of identifying higher-than-expected cases of one or more AESIs after a COVID-19 vaccination, the investigation covering the most commonly used vaccines, the mRNA-based vaccines distributed by Pfizer and Moderna, as well as nonreplicating adenovirus vector-based vaccines. and protein-based vaccines. Overall, it was a massive undertaking that belied the frequent antivax lie that vaccines aren’t studied for safety and efficacy, but somehow antivaxxers tried to spin it as showing the vaccines to be horribly dangerous.
Unsurprisingly, they’re at it again.
How antivaxxers spin a vaccine safety study
Let’s take a look at the headlines and spin by antivaxxers, after which I’ll look at the study itself. First up, The Epoch Times, whose headline reads Elevated Risk of Epilepsy, Appendicitis in Children After COVID-19 Vaccination: Study and which begins:
Children who received the AstraZeneca or Pfizer-BioNTech COVID-19 vaccines faced an elevated risk of epilepsy and appendicitis, according to a new study.
Pfizer recipients were also more likely to suffer from demyelinating disease or heart inflammation, researchers found.
Dr. Julia Hippisley-Cox, a professor of clinical epidemiology at the University of Oxford’s Nuffield Department of Primary Health Care Sciences, and colleagues obtained data from a national database on COVID-19 vaccination, mortality, hospital admissions, and COVID-19 infections. They wanted to look at the link between COVID-19 vaccines from AstraZeneca, Pfizer, and Moderna with 12 outcomes, including the heart inflammation condition called myocarditis.
Sounds alarming, right? Also notice that the article says nothing else about the other findings of the study, nor does it say anything about the magnitude of the increased incidence found, either relative or absolute, not to mention that it doesn’t say anything about the benefits also reported by the study. (Again, I’ll get to that in a moment.)
Unsurprisingly, one of Mike Adams’ minions, “Lance D. Johnson,” over at Natural News goes even further, with a headline Study finds increased risk of epilepsy, severe allergic shock, myocarditis and appendicitis in children injected with COVID-19 vaccines. The beginning of the post is a dump of antivax talking points thrown in with the cherry-picked, context-free findings of the study:
A recent study, analyzing records from over 5 million children, revealed that those who received either the AstraZeneca or Pfizer-BioNTech COVID-19 vaccines faced increased risks of epilepsy and appendicitis. Additionally, recipients of the Pfizer vaccine showed higher probabilities of demyelinating disease and heart inflammation.
Dr. Anthony Fauci, the former Director of the National Institute of Allergy, and Infectious Diseases (NIAID), quickly recommended COVID-19 vaccines for children in the United States. In the UK, the Medicines and Healthcare products Regulatory Agency (MHRA) began distributing the vaccines to children as quickly as they could be approved.
As these so-called vaccines became available for younger age groups, public health leaders emphasized the importance of vaccinating children to not only safeguard their health but also to contribute to broader community immunity against COVID-19. Dr. Fauci’s recommendations were parroted by the “experts” at the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO), which endorsed COVID-19 vaccination for children as young as six months old.
Today, the CDC has the COVID-19 vaccines on the childhood vaccine schedule, right along with 70+ doses contained in various combination vaccines that are now suspect in the chronic disease epidemic that is ravaging American children.
Notice that last bit, which is nothing more than a very old antivax talking point, in which antivaxxers count individual components of combination vaccines (e.g., MMR, which contains measles, mumps, and rubella vaccines, and DTaP, which contains diphtheria, tetanus, and pertussis vaccines) as a vaccine and then adds up every dose recommended to come up with a number as high as they can, even if they have to distort, in order to make it seem as though children get an unreasonably large number of vaccines in their first 18 years of life. Never mind that this is a huge distortion and that there is no evidence that vaccines are responsible for a “chronic disease epidemic” among our children.
Unsurprisingly, the result of this sort of spin was a flurry of antivax postings on various social media pointing to the study as evidence that COVID-19 vaccines are dangerous for children:
I could go on, but you get the idea. The spin has been to portray this study as evidence that vaccines are more dangerous than the disease in children, just as antivaxxers did with the large safety study in adults published in February. Let’s take a look at the study itself.
What a study concludes vs. what antivaxxers say
As I approached this section of my post, I was half tempted just to cite this exchange:
Of course, me being me, you know that I just can’t stop there. So let’s look at the study by Copeland et al, entitled, Safety outcomes following COVID-19 vaccination and infection in 5.1 million children in England. One advantage—among many—of having a single payer government-funded health insurance plan that covers all citizens is that, unlike the case in the US where health records and databases are fragmented, there is generally a single database that can be mined for health outcomes like this. In this case, the investigators used the English National Immunisation Management Service (NIMS) database of COVID-19 vaccination, whose records are linked at the individual-level to national data for mortality, hospital admissions, and SARS-CoV-2 infection. Using this database:
We undertook a self-controlled case series design, originally developed to examine vaccine safety36,37, to investigate the association between COVID-19 vaccines available in the UK between 8th December 2020 and 7th August 2022 (BNT162b2, mRNA-1273 and ChAdOx1) and hospitalisation with the following pre-specified outcomes: myocarditis21,22, MIS-C38, immune thrombocytopenia (ITP)39, epilepsy40, acute pancreatitis41, acute disseminated encephalomyelitis (ADEM)42, Guillain-Barre syndrome43, appendicitis44, demyelinating disease6, myositis45, angioedema46 and anaphylaxis46. We also investigated the association of SARS-CoV-2 infection with these outcomes in children who had been vaccinated prior to infection compared to those who were unvaccinated at time of infection. We compared the incidence of hospitalisation from each outcome in the six weeks following vaccination or SARS-CoV-2 infection relative to the baseline period, and estimated the absolute risk as the excess number of events expected per million children exposed. We also conducted a matched cohort analysis using vaccinated and unvaccinated children included the QResearch primary care database to improve the robustness of the study.
Here’s a bit more on the methodology regarding outcomes of COVID-19 infection in vaccinated versus unvaccinated children:
The cohort included all children aged 5–17 years who had received at least one dose of BNT162b2, mRNA-1273 or ChAdOx1 vaccine or who had a positive SARS-CoV-2 test between 8th December 2020 and 7th August 2022. In each self-controlled case series analysis, we only included children who were admitted to hospital or died from the outcome during the study period and excluded children with a hospitalisation for the same outcome in the two years prior to 8th December 2020 and those who received other COVID-19 vaccine types. We also undertook an analysis in young adults aged 18–24 years as a comparison.
Personally, I think it’s informative to compare what Mike Adams’ minion says to what the study actually finds; this to me is the best way to show how deceptive the spin on the study is. First, however, I like to cite this infographic provided by the investigators that summarizes the findings in terms of excess incidents per million children for the prespecified adverse events examined:
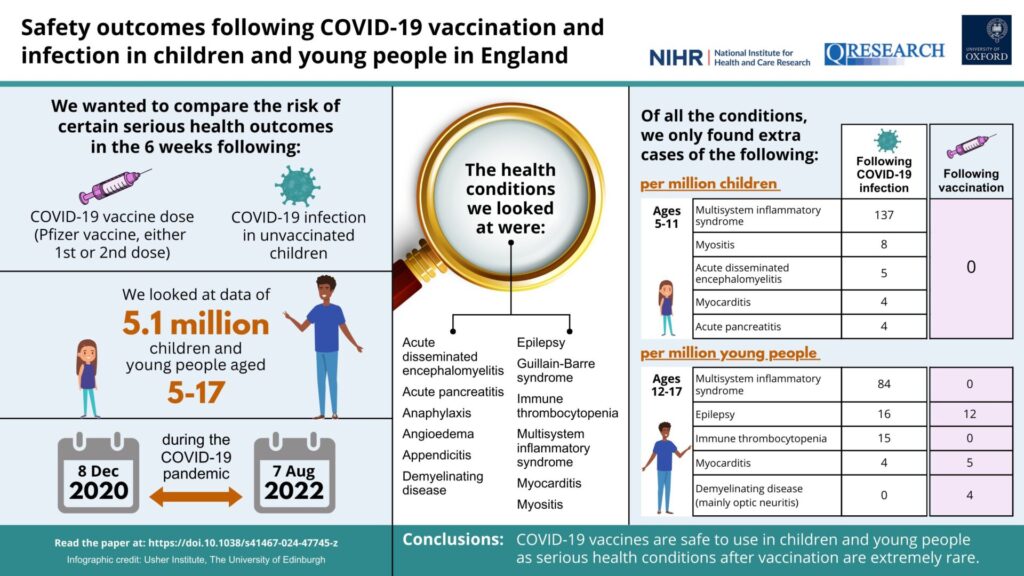
Before I move on, I was curious about the appendicitis, which was hyped in the headlines of both Natural News and The Epoch Times as a risk found from the vaccine. If you read the abstract of the study and peruse the infographic, you’ll see that it’s not included as a major finding, which led me to suspect some picking of cherries, if you know what I mean. If you read the whole study, down to the discussion, you’ll find out why, as the authors conclude:
This study identified two strong safety signals in adolescents associated with the ChAdOX1 vaccine: appendicitis and epilepsy. A substantially increased risk of appendicitis was observed in adolescents following a second dose of ChAdOX1, with an additional 512 (95%CI 283–599) cases expected per million. This estimate is based on a small sample size as the ChAdOX1 vaccine was not approved for use in under-40s in the UK from April 20213,53. Additionally, the increased risk was not identified in the matched cohort study, suggesting the evidence from this study for a causal association between appendicitis and ChAdOX1 vaccination is weak. Appendicitis was highlighted as an outcome of interest for vaccine safety by the US Food and Drug Administration following a clinical trial of BNT162b2, which reported a higher number of appendicitis cases in the vaccine arm compared to the placebo arm44. However, subsequent evidence from observational studies and adverse event reporting databases is conflicting, limited to adults and primarily focusing on mRNA vaccines11,54,55.
Let’s look at Table 4 from the paper, which is one of the “money” tables in terms of summarizing findings:

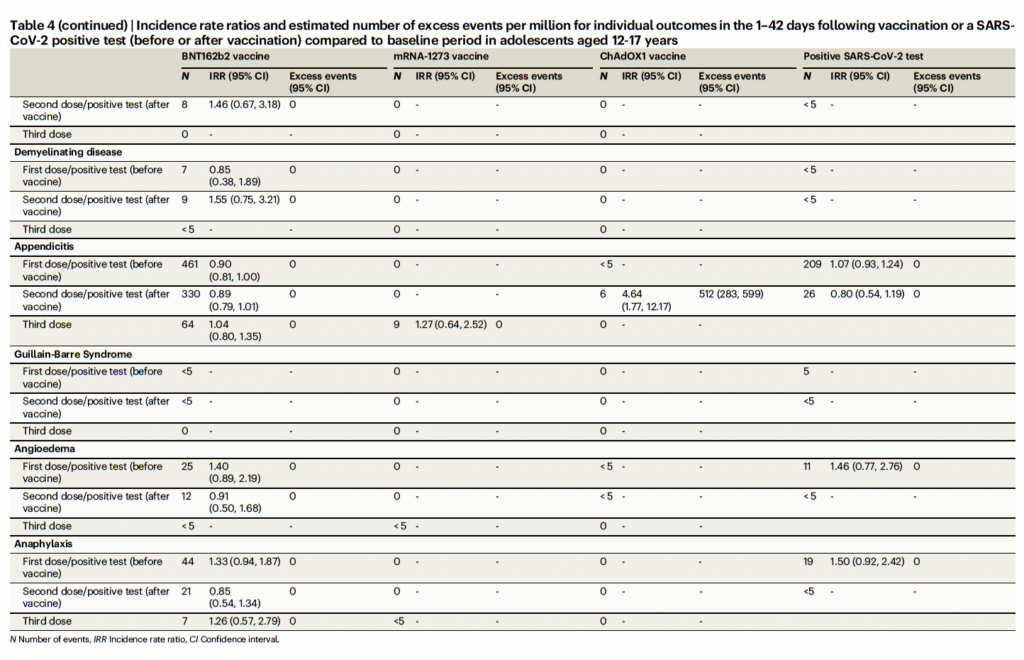
In other words, the numbers were small, the 95% confidence intervals were wide, the elevated risk was only seen for the AstraZeneca vaccine in adolescents after a second dose, and the finding wasn’t consistent (the matched cohort study didn’t detect it), all of which is why the finding was mentioned and discussed but not highlighted. None of that stops Natural News:
Moreover, females who received AstraZeneca’s shot faced a significantly higher risk of hospitalization with epilepsy and appendicitis. The data on the Moderna vaccine was incomplete because the vaccine was not widely distributed to UK children.
Note the selectivity. But what about epilepsy, since that was examined too? Well, antivaxxers fail to mention this part of the analysis, in which the authors stated that they thought it unlikely that their observations indicated a causal effect:
We also observed a modest increased risk of hospitalisation with epilepsy in adolescents following a second dose of BNT162b2, with an additional 12 (95%CI 0-23) cases estimated per million exposed in 12–17-year-olds. However, a diagnosis of epilepsy is made over a period of time as it typically involves outpatient referral, a magnetic resonance imaging (MRI) scan and an electroencephalogram47. Therefore, this reported increased risk of epilepsy is highly unlikely to reflect new-onset epilepsy triggered by the vaccine. A limitation of the data was that we were only able to exclude prior hospital admissions with epilepsy (or any of the pre-specified outcomes) in the two years preceding the study start date. Therefore, the increase in admissions reported here is more likely to reflect seizures in children with an already underlying diagnosis of epilepsy or other chronic neurological condition but who hadn’t been hospitalised in the previous two years, which we were unable to capture in our dataset, rather than new diagnoses.
And later:
Following a first dose of ChAdOX1, we observed a substantially increased risk of hospitalisation with epilepsy, particularly in females, with 813 (95%CI 44–1164) excess cases estimated per million female adolescents vaccinated with a first dose of ChAdOX1, but as discussed above, these are not likely to reflect new diagnoses of epilepsy. The small sample size (only 0.3% of adolescents received ChAdOX1 for the first dose) and resulting wide confidence interval for this estimate should be noted. We also found that there was a higher proportion of adolescents with a hospital admission for epilepsy in the two years prior to the study start date who received ChAdOX1 for the first dose (2.7%), compared to BNT162b2 (0.2%) and mRNA-1273 (0.6%). While these individuals were excluded from the analysis, it is indicative that a higher proportion of adolescents who received a ChAdOX1 vaccine were included in a priority group, such as those with a chronic neurological disease including epilepsy3, compared to those who received mRNA vaccines and that the majority of hospitalisations with epilepsy following ChAdOX1 were likely in adolescents with a pre-existing condition. We did not identify an increased risk of hospitalisation with epilepsy following vaccination with ChAdOX1 in the matched cohort study. Our findings, together with a recent study that found evidence for increased risk of cardiac death in young women following a first dose of non-mRNA vaccine56, suggest that further work would need to be done to ensure the safety of ChAdOX1 in young people if it were to be used in future vaccination programmes.
In other words, the increased risk of hospitalization with epilepsy or seizures might be associated with the vaccines, but is small and could well be restricted to children with pre-existing epilepsy, whether diagnosed or not. Moreover, as antivaxxers are won’t to do, even this small increased risk, if real, is not compared with the risk of the disease. Indeed, the number of excess cases of epilepsy after infection is higher (16 per million) than after vaccination (12 per million).
As for myocarditis, which is the one adverse event that is clearly associated with the mRNA vaccines in adolescents, the authors found:
In our cohort of > 2.8 million vaccinated adolescents aged 12–17 years, we estimated 3 (95%CI 0–5) and 5 (95%CI 3–6) excess cases of myocarditis per million exposed in the 1–42 days following a first and second dose of BNT162b2, respectively. We did not observe an increased risk of myocarditis following vaccination with mRNA-1273 in adolescent males or females as some previous studies have reported23,24,25,26,27,28,29,30,31,32. However, the mRNA-1273 vaccine was only given to a relatively small number of adolescents in England, primarily for the third booster dose, therefore the study was underpowered to detect statistically significant associations, except for very large effect sizes. As expected, we observed a substantially increased risk of myocarditis in the 1–42 days following almost all doses of both mRNA vaccines in young adults aged 18–24 years compared to the baseline period, consistent with other studies reporting an increased risk of myocarditis in young adult males following a second mRNA-1273 vaccine dose24,25,26. Importantly, however, there were no deaths following a diagnosis of myocarditis in under-18s, and our findings are likely to reflect self-limiting disease.
In other words, the authors’ findings are consistent with what we already know about myocarditis following vaccination; it’s generally mild and self-limited.
Now, let’s look at what the authors of the study concluded:
In summary, we found no strong evidence for increased risks of 12 pre-specified vaccine safety outcomes following COVID-19 vaccination in children aged 5–11 years and no new significant safety concerns in 12–17-year-olds following vaccination with mRNA vaccines recommended for use in these age groups in the UK by the JCVI. Additionally, in unvaccinated children we found increased risks of hospitalisation from seven adverse outcomes including MIS-C and myocarditis following SARS-CoV-2 infection that were either not observed, or were reduced, following vaccination. Overall, our findings support a favourable safety profile of COVID-19 vaccination using mRNA vaccines in children and young people aged 5-17 years.
I agree. This study is further evidence of a good safety profile for COVID-19 vaccines among children. Of course, if you’re an antivaxxer, there can only be one reason why the study authors conclude that the safety profile is favorable, and I bet you can guess what it is:
The authors of the study had previously served on various UK and Scottish Government COVID-19 advisory groups that recommended COVID-19 vaccines for children. The authors, funded by the National Institutes of Health, also enjoy financial ties to Moderna and AstraZeneca.
This could be the reason why the authors provided a perplexing conclusion to the study’s results. The authors said that their findings “support a favorable safety profile of COVID-19 vaccination using mRNA vaccines in children and young people aged 5-17 years.” This mockingly dangerous conclusion should put all their careers in jeopardy, considering that the study found an increase in hospitalizations because of the COVID-19 vaccines. The lead author, Dr. Julia Hippisley-Cox, did not comment on the study.
Of course, Mike Adams’ minion thinks that the only reason why the investigators who did this study concluded that the risk-benefit profile for COVID-19 vaccines is favorable in children must be because they are in the pay of big pharma. I did, however, briefly wonder if I should have looked at the COI statement at the end of the study before I poo-pooed Adams’ minion too much. So I did:
This research is funded by the NIHR School for Primary Care Research, Grant Reference Number 622. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. We acknowledge the contribution of EMIS practices who contribute to QResearch and EMIS Health and the Universities of Nottingham and Oxford for expertise in establishing, developing or supporting the QResearch database. This project involves data derived from anonymised patient-level information collected by the NHS. The SARS-CoV-2 test data were originally collated, maintained and quality assured by Public Health England (PHE) and transferred to NHS England during the study. Access to the data was therefore facilitated by NHS England. The Hospital Episode Statistics, Secondary Users Service (SUS-PLUS) datasets and civil registration data are used by permission from NHS England who retain the copyright in that data. NHS England and Public Health England bears no responsibility for the analysis or interpretation of the data. JHC is supported by an NIHR senior investigator award. NLM is supported by a British Heart Foundation Chair Award (CH/F/21/90010), Programme Grant (RG/20/10/34966) and Research Excellence Award (RE/18/5/34216). DPJH is supported by the Wellcome Trust (215621/Z/19/Z), Medical Research Foundation and UKDRI (principal funder UKRI Medical Research Council).
So this research was funded by a grant from the UK National Institute for Healthcare Research. If the authors have financial ties to the pharma companies and it wasn’t mentioned in the Acknowledgments section as a conflict of interest, that would be something, for sure, but thus far I haven’t been able to find evidence of Julia Hippisley-Cox or any of the other authors having financial ties with Moderna and AstraZeneca and will say outright that, if any of the authors do have such ties, they should have been disclosed in the Acknowledgments section.
My guess, though, is that these “ties” are the sorts of guilt-by-secondary association “ties” that antivaxxers love to use, such as when Mike Adams falsely accused me of being in the pocket of Sanofi-Aventis because I was doing research using a drug made by the company and my university had accepted research grants from Sanofi-Aventis. I will, however, accept correction if I missed something, unlike Mike Adams, who would double down.
Antivaxxers like to claim that vaccine researchers don’t bother to look for adverse events due to vaccines, when in fact this study and the study that I discussed in February are very strong evidence that public health officials and vaccine researchers are very much interested in carefully assessing the risk-benefit profiles of vaccines, to the point of investing considerable resources to study outcomes in millions of adults and children. What antivaxxers really don’t like is that the results of these large studies consistently fail to support their fear mongering portraying vaccines as massively dangerous compared to the disease, and ineffective, to boot. They will weaponize any large study that carefully and honestly reports adverse events from vaccination, ignoring the magnitude of the risks (almost always very small) versus the benefit (significantly greater than the risks).

