Vitamin D has a long history of use as a medical treatment of osteoporosis. While observational studies have linked low vitamin D blood levels to a range of illnesses, prospective trials that have examined the medical benefits of vitamin D supplementation have not been impressive. While there have been some high-quality, large prospective trials using vitamin D as a therapy, there are also a huge number of smaller, poor-quality trials, many of which have produced positive results that haven’t been replicated in larger studies. The net effect has been lots of positive attention, but some persistent questions about whether supplementation is useful or beneficial. While testing is widespread, optimal serum concentrations for skeletal health and broader health benefits are controversial, and have not been clearly defined. Now a new randomized controlled trial examines the cardiovascular effects of monthly doses of vitamin D.
Why another trial of vitamin D?
Heart disease and stroke are leading (and growing) causes of death, especially in older individuals. Vitamin D acts on cells throughout the vascular system, reducing inflammation, regulating the renin-angiotensin-aldosterone system, and inhibiting vascular smooth muscle proliferation. Observational studies have shown an inverse relationship between vitamin D levels and cardiovascular disease risk. Prospective controlled trials, on the other hand, have produced mixed results. Some studies indicate an inverse association with cardiovascular disease risk at certain vitamin D levels, while others found no significant association. Consequently, there is ongoing interest in verifying if vitamin D can reduce the risk of disease.
A meta-analysis of randomized controlled trials concluded that vitamin D supplementation did not prevent cardiovascular events. However, that analysis was predominantly based on the Women’s Health Initiative Trial, which had limitations in terms of participant characteristics, dosage of vitamin D, and compliance. The Vitamin D Assessment (ViDA) study and the Vitamin D and Omega 3 trial (VITAL) also found no effect on cardiovascular disease outcomes, but they both had noted limitations.
The D-Health Trial was a large study involving 21,315 older Australians. Previous analyses of the trial showed no reduction in all-cause mortality or cardiovascular mortality with vitamin D supplementation. However, the impact on major cardiovascular events specifically had not been analyzed. In this current study, we examined data from the D-Health Trial to determine if monthly vitamin D supplementation (60,000 IU) affected the incidence of major cardiovascular events in Australians aged 60 years and above.
The D-Health Trial
The D-Health Trial was a randomized controlled trial with two groups: vitamin D recipients (60,000 IU of vitamin D3 (cholecalciferol)), or placebo, taken as once monthly doses. The trial included adults aged 60-79 years from across Australia. Every year, participants were sent 12 tablets, and reminded to take them monthly by text, email or landline call. Participants were selected randomly from voter registration, which is mandatory in Australia. Additionally, individuals aged 60-84 years were also recruited through media coverage and existing participants’ contacts. Individuals with specified conditions, (hypercalcaemia, hyperparathyroidism, kidney stones, osteomalacia, sarcoidosis) or those who already consumed more than 500 IU of vitamin D per day were excluded from the trial. Participants in the study provided information about their demographics, lifestyle, health conditions, and vitamin D intake through a baseline questionnaire.
Participants were asked annually to report the number of study tablets they consumed, and if they took any other vitamin D supplements outside of the study. Adherence was assessed by comparing the number of tablets taken to the total number they should have taken if fully adherent ( 60, except for those who died during the trial). Participants were encouraged to limit their use of vitamin D supplements not provided by the study, but were allowed continue in the trial as long as that intake did not exceed 2000 IU per day. (This would keep intake below 4000 IU per day.)
Cardiovascular events were one of the planned outcomes in the D-Health Trial, along with 44 other outcomes. The main outcome was the occurrence of the first major cardiovascular event, (myocardial infarction, stroke, or coronary revascularization). Secondary outcomes included the first myocardial infarction, stroke (total, ischemic, and hemorrhagic), and coronary revascularization. Events were determined from linked hospital data, medicare benefits data, and mortality records, which was very comprehensive owing to Australia’s universal health insurance system. Australia’s Pharmaceutical Benefits Scheme has data on prescription drug use and was used to determine use of statins and other cardiovascular drugs.
The sample size was determined to ensure 80% power to detect a 9% difference in the mortality rate, with a type 1 error rate of 0.05. Based on the available sample size for this analysis (n=21,302), the authors estimated they would have 80% power to detect a 16% difference in the incidence of the first major cardiovascular event. Participants were followed from the time of randomization until the earliest occurrence of a major cardiovascular event, the last known date of being alive, five years and one month after randomization, or December 31, 2019 (the date which hospital data were available for all states).
Over 21,000 participants were recruited and participated. At the end of the five-year trial, the majority of participants (79%) were still taking the study tablets. The percentage was similar between the vitamin D group (80%) and the placebo group (78%). Before completing the intervention period, 866 individuals died. The average duration of treatment was five years, and over 80% of participants reported taking at least 80% of the study tablets. This adherence rate was consistent in both the vitamin D group (84%) and the placebo group (82%).
During the trial, the mean serum 25(OH)D concentration was 77 nmol/L (with a standard deviation of 25) in the placebo group, while it was 115 nmol/L (with a standard deviation of 30) in the vitamin D group. The incidence of adverse events was comparable between the two groups.
Overall, there were a total of 1,336 major cardiovascular events. Among these, 637 occurred in the vitamin D group (6.0%) and 699 in the placebo group (6.6%). The rate of major cardiovascular events was slightly lower in the vitamin D group compared to the placebo group, with a hazard ratio of 0.91 (confidence interval 0.81 to 1.01). Given the confidence interval crossed one, this means that there was no statistically significant effect. On average, 172 participants would need to be treated (NNT) with vitamin D supplementation to prevent one major cardiovascular event. The confidence interval for this estimate (not provided by the authors) is 82 to infinity. If 1,000 people aged 60-84 in this group of participants took a placebo, 66 will have a cardiovascular event. If the same group took, vitamin D, that might drop to 60. Or there may be no difference.
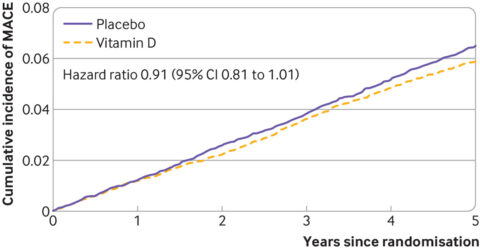
Figure 2: Cause specific cumulative incidence of major cardiovascular events according to randomisation group and time since randomisation.
When you dig into the hazard ratios for the individual events, there are some statistically significant differences (e.g., heart attack, which is barely significant). At this point this feels a bit like dumpster diving for positive results, so I’ll leave a link to that data here if you want to peruse it.
Conclusion: Vitamin D likely offers no cardiovascular benefits
We already know from this same data set that vitamin D had no effect on all-cause mortality. This well done clinical trial offers modest evidence that supplementation may reduce the incidence of some cardiovascular events. However, in aggregate, no benefit was show on overall cardiovascular event occurrence. When considered in the context of accumulated research to date that has showed vitamin D has no measurable benefit, it is difficult to consider even these very weak findings to be very compelling. We do know that vitamin D is inexpensive, and that there is low risk of toxicity. But this trial adds to the body of evidence showing no cardiovascular benefits to supplementation.