If there is one aspect of “alternative” medicine that both critics and fans should agree on, it’s that products should be manufactured to high standards. What’s on the label should accurately describe what’s in the bottle. Product quality standards are essential, whether you’re using herbs or drugs. And when it comes to ensuring the products we buy are of high quality, we’re all effectively reliant on regulation to protect us. As a pharmacist, I can’t personally verify that each tablet in your prescription contains the active ingredient on the label. I am dependent on a supply chain that may stretch around the world. While the product manufacturer may be reputable, it’s only a regulator that can realistically verify and enforce production to strict quality standards. The same cannot be said for products like supplements and herbs which are regulated differently than drugs, and held to different, and in some cases, weaker standards. A weak regulatory framework, which doesn’t hold manufacturers to account, would be expected to result in a product of lower quality. And that’s exactly what you see when you look at supplements on the market today.
A new study paints a disturbing picture of the quality standards of herbal products commercially sold in Canada and North America. Entitled “DNA barcoding detects contamination and substitution in North American herbal products”, Steven G. Newmaster and colleagues from the University of Guelph in Ontario conducted authenticity testing of 44 herbal products. The authors have no personal bias against herbal medicines – the study was funded by non-commercial interests, and none of the authors reported any conflicts of interest. They’re actually quite positive about the therapeutic effects of herbal medicines, noting “there is considerable evidence of the health benefits of herbal medicine,” a statement that most at SBM would take issue with. The authors note a significant problem in the way these products are produced and distributed:
There are currently no best practices in place for identifying the species of the various ingredients used in herbal products. This is because the diagnostic morphological features of the plants on which the current Linnaean taxonomic system is based cannot typically be assessed from powdered or otherwise processed biomaterials. As a result, the marketplace is prone to contamination and possible product substitution, which dilute the effectiveness of otherwise useful remedies, lowering the perceived value of all related products because of a lack of consumer confidence in them. Herbal product substitution has been documented for many individual medicinal plant species, teas, and ‘nutraceuticals’. Although there is limited research available, the frequency of product mislabeling in herbal products has been estimated at 14% to 33% from previous studies. There are legitimate health concerns for consumers which results in a lack of confidence in safe, high quality herbal products.
Before we go into the study, it’s worth distinguishing between the physical characteristics of drug products and medicinal herbs. A surprising number of drugs we use today are derived from, or based on, chemicals originally found in nature. Acetylsalicylic acid (aspirin) is derived from salicin found in the bark of the willow tree. The antimalarial quinine is found in the bark of the Cinchona tree. Atropine was found in nightshade, and digitalis (digoxin) is what makes consuming the foxglove plant toxic. All had their origins as natural remedies. The benefit of drug products over herbs is the reproducibility of effects, which starts with isolating and purifying the active ingredient. The next step is manufacturing a dosage that results in consistency in absorption and standard and predictable dosages. Clinical trials establish if the drug has meaningful effects. So when I’m speaking with a patient about their new prescription drug, I know there’s a traceable relationship between the characteristics of product they’ve been dispensed and the drug product that was studied in the clinical trials. Consequently, it’s reasonable to expect the same outcomes and effects, based on this linkage. Even doing something as simple as changing the product from a tablet to a capsule may result in a regulator requiring bioequivalence study to confirm the effects are the same.
The rigorous controls in place for drug products don’t carry over to supplements. Many herbal products that are sold as “medicinal” contain a variety of chemical ingredients, several of which may have medicinal effects. If there was a clinical trial conducted, there is no simple way to verify that other versions of the plant contain the same combination of ingredients that produced the effects. Some herbal products do “standardize” one or more chemical constituents, where the suspected active ingredient is measured and verified in production. But even this isn’t an assurance of quality. Feverfew is a herb used to prevent and treat migraine, which is usually standardized to its content of the chemical parthenolide , the suspected actual ingredient. Yet there are dozens of chemical components in the feverfew leaf, and even standardized products cannot demonstrate effectiveness, which could mean that the active ingredient isn’t the parthenolide. Or it could be that feverfew simply doesn’t work. Without the purification and standardization offered by finding, isolating, and then testing the active ingredient, herbal products will always be at a disadvantage when it comes to quality, consistency, and predictability of effect.
Once we swallow a substance, be it a herb or a drug, our body can’t distinguish the difference. Chemicals are absorbed into the bloodstream, circulate in the body, and presumably reach the site of action and then have some sort of biological effect. They are eventually excreted, sometimes only after being transformed in the liver. The more consistent the original product, the more predictable the response in the body. Given the similarities of how herbs and drugs behave in the body, it’s hard to understand the rationale for a completely different regulatory standard for herbs. In the USA, it’s the Dietary Supplement Health and Education Act (DSHEA) that creates a double standard. In Canada, it’s the Natural Health Products Regulations. They are similar in that they implement some manufacturing and safety standards, while essentially eliminating the requirement to demonstrate that the product actually does anything useful. DSHEA goes so far as to put the requirement to demonstrate harm on the FDA, instead of requiring the manufacturer to demonstrate that a product is safe and effective. Canada regulates differently, but to an equally low standard. As I’ve pointed out before, the Natural Health Product Regulations have had a similar effect at creating a manufacturer-friendly market: Pretty much anything goes, and even homeopathic cancer, homeopathic corn, and homeopathic water (think about that one) have been approved by Health Canada as “safe and effective”. And when it comes to herbs, Health Canada approves products that are said to “tonify the kidney and fortify the yan“.
The separate regulatory systems for natural products, supplements and herbs in Canada and the United States have led to a boon in the development and sale of these products. The barriers to establishing a business are trivial compared to drugs. Importantly, there’s no requirement to actually demonstrate your product works. There’s not even a requirement to show your product is consistent batch-to-batch, because to show that you’d first need to demonstrate some objective and measurable effects. Finally and most importantly, there’s really no simple way for a regulator to actually test to see if what you’re putting in the capsule (for herbs) is actually what you say. Until now, and the technology used by Newmaster. It’s called DNA barcoding, which essentially treats DNA strands like UPC codes, examining and distinguishing between different species.
The researchers purchased herbal products for sale in Toronto, Canada and via mail order from the United States. Twelve manufacturers were involved, representing 44 products (41 capsules, 2 powders, 1 tablet). They also obtained 50 samples of known products from greenhouses. Both were studied in a blinded fashion. In total there were 30 herbal species, and these were studied against a barcoded “library” of over 100 species. Their definitions were as follows:
- Authentic: a product contained the DNA barcode for a species that was the main ingredient on the label.
- Contaminated: contains labelled ingredient and also the DNA barcode for a species not on the label.
- Substitution: a product contained the DNA barcode for a species not on the label, and there was no trace of the labelled ingredient.
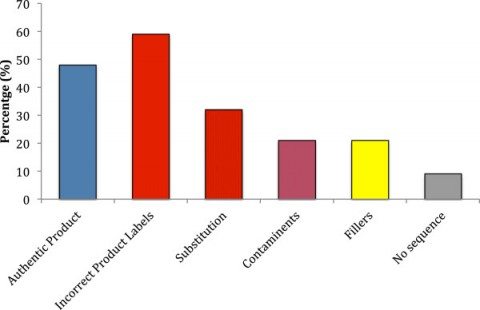
Here were the results:
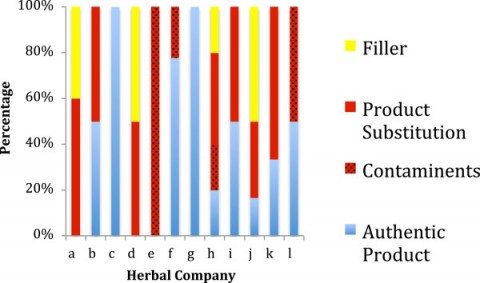
The findings are dismal and quite discouraging for consumers and health professionals alike. Only 48% of products were authentic. 59% of the products contained species not listed on the label. One-third contained fillers and contaminants that were not labeled. Amazingly, another third of products tested contained a substituted ingredient – and none of the labeled ingredient. Of the 12 companies sampled, only two had authentic products without any substitution, contaminants, and fillers. Unlabelled fillers including wheat, soy, and rice, were found in 20% of products. Three companies had products for which no products could be authenticated:
Some of the products were of such poor quality that harms could be expected. One sample was labelled as containing St. John’s wort (used to treat depression) but actually only contained senna, a laxative. Several products were contaminated with feverfew, which has chemical ingredients that can cause side effects, including withdrawal syndromes. Feverfew may also interfere with the elimination of some prescription drugs, and needs to be avoided in pregnancy. Another product was contaminated with black walnut, which has a nasty side effect profile. And there’s also the undisclosed wheat in some products, which would be harmful to those with Celiac disease or a wheat allergy.
This is far from the first sign that there are issues with supplement quality standards. Earlier this year I wrote about the frequency and characteristics of dietary supplement recalls in the USA. That study noted that every supplement recalled between 2004 and 2012 was due to unapproved ingredients. But recalls don’t give us a comprehensive summary based on sampling – which is where this study excels. Other studies have raised similar concerns:
- A study of 131 herbal teas revealed only 58% of products could be authenticated, and 33% were contaminated.
- A study of 40 “black cohosh” supplements noted that 25% were substituted with related species.
- Half of the ginseng products examined in one study contained other forms of ginseng than the labelled Panax ginseng.
- A 2005 survey of 230 Ayurvedic medicines found that 20% were contaminated with heavy metals such as lead, mercury and arsenic.
The implications
Without reliable, consistent products, consumers can’t use herbs safely, or even assume they might work as expected. Health professionals are in a similar bind, and can’t make recommendations without high-quality products to rely on. For my entire career I’ve cautioned consumers about the quality concerns with herbal products and supplements, and the resultant risks that make me very hesitant about their use. And in the absence of good evidence of benefit, the potential risks take on more importance. There’s no question that legislation like DSHEA has created a tremendous business opportunity for manufacturers. The market is comparatively easier for manufacturers to enter. Combine this with little requirement to test products, or substantiate claims they even work, and you get a market where product contamination and substitution appears commonplace. It’s exactly what you’d predict if you loosened or eliminated the regulation of prescription drugs. Supplements do share one key feature with prescription drugs: this is big business, a $28 billion market in the US in 2010, with year-over-year growth exceeding 5% annually for the past several years. And the industry is actively behaving in ways that don’t favor consumers, such as fighting moves to give consumers more information about their products.
Perhaps not surprisingly, the supplement industry rejects this latest study, raising some minor criticisms (e.g., asking for reproducibility) but also handwaving away the overall conclusions, with no acknowledgment of the serious nature of these findings. If herbs actually have medicinal effects, then contamination of this nature and scale should be unacceptable within the industry. It’s a disappointing response, because if there’s a single issue that threatens true science-based use of herbs and supplements, it’s product contamination and adulteration worries. And that’s exactly what’s starting to occur. Last month the Children’s Hospital of Philadelphia announced it was no longer supplying supplements, and would discourage patients’ families from their use. Why?
’Because vitamins and dietary supplements are essentially unregulated, there is no sound information about adverse side effects, drug interactions, or even standard dosing for the vast majority of them,” said Sarah Erush, CHOP Pharmacy Clinical Manager and a member of the hospital’s Therapeutic Standards Committee, which recommended the policy change that was rolled out over the summer. “Administering these medications — particularly to children with serious health complications — is unethical when the risks are unknown, and when there are alternative treatments that have been proven in clinical trials to be safe and effective,” Erush said.
Conclusion
This new study joins several others in pointing out serious quality concerns with herbal products sold today. Substantive improvements are necessary to bring the quality of herbal products in line with conventional drug products. And until supplements demonstrate reliable and consistent quality, they cannot be counted on to actually deliver any predictable health effects. Yet without effective regulation to mandate and enforce better standards, there is no external pressure for change. Lax regulation has been a boon to manufacturers, and the supplement industry has exploded. It’s unfortunate that the same conditions actually make it harder to find products that are safe and effective. Until these products are held to more rigorous quality standards, consumers will continue to pay a form of health roulette each time they purchase a herbal supplement.
Reference
Newmaster S.G., Grguric M., Shanmughanandhan D., Ramalingam S. & Ragupathy S. (2013). DNA barcoding detects contamination and substitution in North American herbal products, BMC Medicine, 11 (1) 222. DOI: 10.1186/1741-7015-11-222