
In any medication, only a fraction of the content is actual medicine.
Several years ago I wrote a post on pharmaceutical excipients – the substances that are used to make the tablets, capsules, creams, ointments and liquid dosage forms of supplements and drugs. Excipients have traditionally been defined as “inert” ingredients, used to package and deliver the active pharmaceutical ingredient (the “API”) into the body in a consistent, reproducible, and sometime palatable manner. Excipients are commonly described in disparaging terms, like “fillers” and “additives” but they actually play a critical role in ensuring that the medications (or supplements) we take are stable and consistent. There are hundreds of ingredients that are approved excipients that can be used in formulating a dosage form.
Recently a paper caught my eye that’s led me to revisit this topic and do a bit of a deeper dive into excipients. Entitled “‘Inactive’ ingredients in oral medications”, it’s from Daniel Reker and colleagues, and was published in Science Translational Medicine. The authors seem to get worked up about drug manufacturing characteristics that are not themselves controversial or even problematic, yet may be surprising to non-pharmacists who don’t understand the science, art and practice of pharmaceutics – the science of dosage form design. Pharmaceutics was of those courses that in pharmacy school we rolled our eyes at (when would we ever make acetaminophen tablets?) but the principles of pharmaceutics are largely immutable, even as we’ve reached a point where a single tablet or capsule can cost $500. So I want to touch on a few of the issues that this paper raises as a complement and addition to my 2011 post, and discuss how excipients can sometimes be the cause of adverse reactions.
What are excipients and why do medicines contain them?
Can you remember the last time you took any medication? It was probably a tablet, as tablets are the most commonly prescribed form of medication. The vast majority of each tablet (in terms of mass) is excipients, not active ingredient. The ratio of active pharmaceutical ingredient (API) to excipient varies based on the dosage and the characteristics that are necessary to ensure the dosage form is stable and acceptable, and is delivered into the body (usually the bloodstream) in a predictable manner. If you consider that doses of some medications can be measured in micrograms and miligrams, then it’s clear the tablet will need some additional mass to the tablet just to make a single dose possible to handle. You also need to ensure that each dose is precise, and fillers allow the dose to be distributed consistently in a production batch. You then need binders, which actually hold the tablet together after the doses are manufactured in a press, and ensure they don’t crumble in the bottle or vial. But since you’ve compressed the material, a disintengrant may also be required to rapidly break the tablet apart once it reaches the right part of the digestive system. Other ingredients may be added to help the material flow properly in the production process. Finally, some excipients are added to help tablet identification (colours, shapes, and imprints), to protect the API from light or moisture, or to control the release of the product (e.g., enteric coating). The table below, reproduced from the Australian Prescriber, describes the most common excipients that are present in tablets – and why they are there.
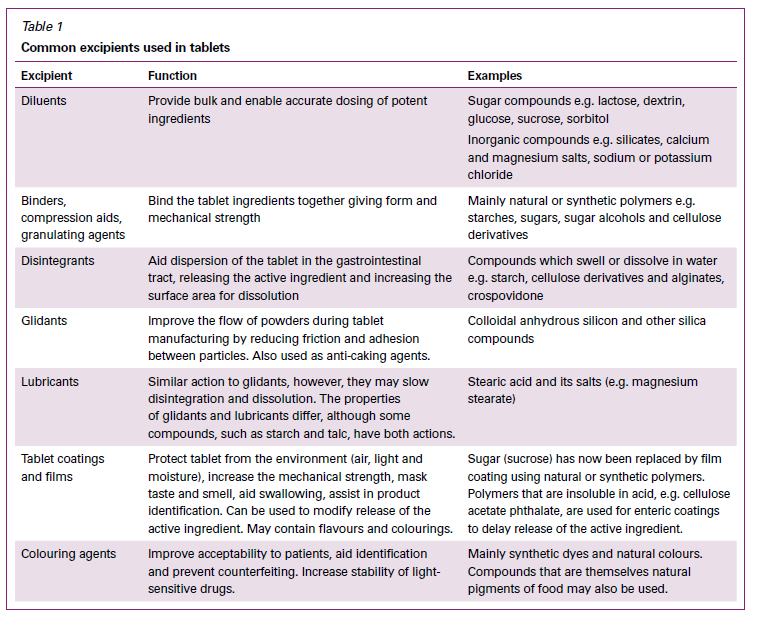
From Australian Prescriber.
It’s conceivable that patients taking several different drugs per day (not uncommon in the elderly) could end up taking a few grams of excipients each day. In most people, excipients are not a problem. In some people, they can be. A small but sizeable proportion (1%-10%) of the population report some form of food allergy or intolerance. Whether or not those reactions are real or simply perceived, they mandate a closer look at the excipients in medications.
Allergies, intolerances, and adverse reactions to excipients
The perfect excipient has no biological effects other than the desired one, is non-toxic, and does not interact with the API or any other ingredient. For many people, excipients are invisible. But some people cannot tolerate specific excipients, and may attribute any untoward effects to the drug itself, not even recognizing that an excipient may be to blame. Importantly, generic versions of drugs may contain different excipients. Some excipients that have been described to cause negative reactions include:
- Starch can be used as a binder, diluent or disintegrant. When starch is extracted from wheat, it can contain trace amounts of gluten. These trace amounts are considered acceptable for those with celiac disease, though some may want to choose other products, regardless of the lack of risk.
- Lactose is a common ingredient in medicines. Some people are lactose intolerant, or may avoid all fermentable oligosaccharides (FODMAPs) (including lactose) to manage symptoms of irritable bowel syndrome. Generally, the amount of lactose is so small in any dosage form that it is unlikely to cause any symptoms or discomfort at all. In those with severe intolerance, other dosage forms or medications may be preferred.
- Eggs can cause allergic reactions and may be present in some vaccines. However, vaccines do not always have to be avoided in the presence of an egg allergy, and local public health units can advise on the acceptability and best practices with respect to specific vaccines.
- Peanuts can cause anaphylactic allergies, and while no medications contain peanuts, some (e.g., intramuscular injections, ear drops, enemas, and topical preparations) contain peanut oil (arachis oil). While pharmaceutical-grade peanut oil should be free of peanut protein, patients with severe peanut allergies should not use products that contain peanut oil. Because of the similarities between peanuts and soy, those with severe allergies to soy should also avoid peanut oil-containing products.
- Shellfish may not be something you think of in medication, but some forms of glucosamine supplements are produced from shellfish shells. There’s no compelling reason to take glucosamine supplements, but if you do, use caution if you have a shellfish allergy. While the allergy is caused by a reaction to the flesh of shellfish, not the shell itself, manufacturers recommend avoidance in the case of severe allergies.
- Colourants like tartrazine are used in medications and have been attributed (though not conclusively linked to) activity and attention issues in children. The is no compelling reason for approved colourants to be avoided, but some may prefer products without these ingredients.
- Some preservatives, like benzalkonium chloride, may be present in dosage forms like eye drops and cause irritation or other allergic reactions.
- Sodium may be an ingredient in products like antacids, which could be taken in large amounts. If necessary for dietary reasons, it may be possible to identify low- or sodium-free alternatives.
- Some ingredients may not cause specific intolerances but may simply be avoided for religious or dietary reasons. Gelatin is an ingredient that is routinely used in capsule dosage forms but comes from animal sources. In some cases, the manufacturer may need to be consulted to verify the source of any ingredients.
- Sweeteners like sorbitol can cause diarrhea if taken in large amounts.
- Ethanol may be found in some liquid dosage forms, but is typically avoided in anything intended for children. Doses are typically small, but in excess, even homeopathy can get you loaded. Alcohol can also react negatively with some prescription drugs.
Conclusion
Medicines and supplements contain a variety of ingredients that are included as part of the manufacturing process to ensure stability, consistency and quality. While these excipients are by definition “inert”, they have the potential to cause adverse reactions in rare circumstances in sensitive individuals. Importantly, excipients can vary between brands of the same product. Detailed information is not always easily accessible. When in doubt, ask your pharmacist or phone the manufacturer directly (ask for “medical information”), who will tell you what excipients are present, or can confirm the presence or absence of any excipient in question.

