[Editor’s note: A recent health issue has prevented Harriet from writing her usual Tuesday morning post; instead, here is an overview of some interesting basic science from PhD candidate Justin Quiles on the impacts and complexities of oxidative stress and its relation to health.]
Most of the health-conscious population can recognize the terms “antioxidant” and “free-radical”. In fact, you may have recently heard these buzzwords (in the absence of much science) when a Bai or Vitamin Water commercial interrupted your favorite television show. If not, maybe you know someone who has used this terminology to absolve themselves from the guilty pleasures of excessive red wine or dark chocolate. All jokes aside, while it is true that sufficient dietary intake of antioxidant vitamins and minerals is essential for optimal human health, emerging research indicates that too much of a good thing is in fact bad – a biochemical phenomenon known as reductive stress.
Redox reactions, oxidative stress and aging
The transfer of electrons between molecules is a fundamental aspect of cellular biochemistry. While oxidation describes a loss of electrons, reduction refers to a gain. Importantly, these complementary processes, collectively known as redox reactions, constantly occur in tandem to maintain life. Indeed, redox biochemistry explains how plants release the oxygen we breathe (photosynthesis), which in turn enables us to transform the food we eat into energy (oxidative phosphorylation).
Paradoxically, molecular oxygen (O2) is both essential and detrimental to human (and other) life. During oxidative phosphorylation, O2 is reduced to H2O as energy is produced in the form of adenosine triphosphate (ATP). However, during this very same process, O2 can accept electrons and become transformed into a variety of reactive oxygen and nitrogen species (ROS/RNS), toxic metabolic intermediates that damage DNA, degrade cell membranes, and impair protein function. Several key environmental and metabolic ROS/RNS byproducts are outlined below:
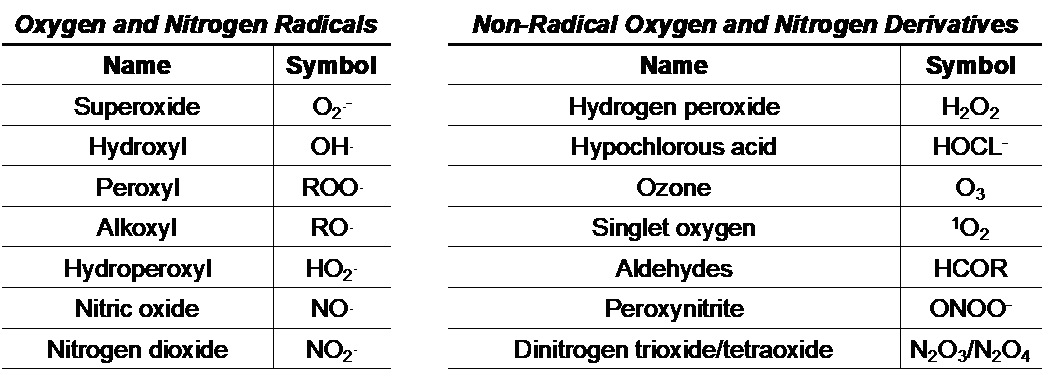
In the 1950s, these abstract chemical reactions reached the forefront of biomedical research as Denham Harman developed his Free Radical Theory of Aging, which suggested that an accumulation of oxygen-derived radicals (namely superoxide) causes or contributes to the cellular damage associated with progressive aging. Over time, this theory has been expanded to encompass additional reactive species that function as pro-oxidants, and has been supported by studies from a number of independent laboratories. While the intracellular sources, relative proportions, and biological targets of ROS/RNS remain an intense matter of debate, nearly six and half decades of research built upon Dr. Harman’s original hypothesis has led to a general consensus that oxidative stress contributes to aging and pathological conditions such as cancer, neurodegeneration, diabetes, and cardiovascular disease.
Exploiting antioxidants to combat oxidative stress
Fortunately, our cells possess an internal defense mechanism to nullify harmful oxidants. These molecules are aptly named the endogenous antioxidants. Encoded within our DNA, endogenous antioxidant proteins can be either enzymatic (i.e. requiring a cofactor for function) or non-enzymatic. In addition to these endogenous molecules, exogenous antioxidants, those not produced within the body, are regularly consumed through a well-balanced diet. A non-exhaustive list of critical endogenous and exogenous antioxidants can be found below (many of which you will likely recognize as vitamins):
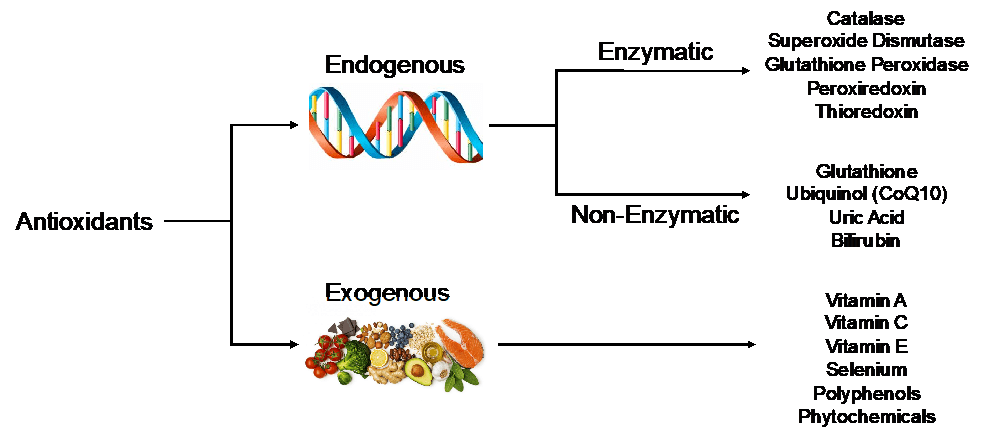
Increasing evidence for oxidative stress in human disease and aging led researchers to develop experimental animal models in which endogenous antioxidant genes were manipulated to enhance their function (i.e. genetic overexpression). Despite promising results in lower organisms such as Caenorhabditis elegans (roundworms) and Drosophila melanogaster (fruit flies), overexpressing endogenous antioxidants in mice, our best non-human model for genetic studies, didn’t help them live longer. However, the overexpression of particular endogenous antioxidants was sufficient to improve health outcomes in mouse models of myocardial infarction (heart attack), diabetes, stroke, and Alzheimer’s disease (among others) suggesting that strategies designed to increase antioxidant abundance during these disease processes could potentially provide a level of protection in humans.
These observations in mice led to a battery of clinical trials in which varied combinations and proportions of exogenous antioxidants (most frequently vitamins) were administered to diverse patient populations in an effort to prevent or treat cancer, cardiovascular disease, and macular degeneration. Sadly, despite massive efforts involving thousands of patients and millions of dollars in grant money, the outcome of these trails was emphatically abysmal. As summarized by the National Institutes of Health (NIH), the primary funding agency for much of this work, antioxidant supplementation did not reduce risk of heart attack, stroke, cataracts, and age-dependent cognitive decline. In fact, in two separate studies, exogenous antioxidant supplementation actually increased the incidence of lung and prostate cancer.
So, why did antioxidant supplementation fail these patients? While there is no clear-cut answer to this question, the NIH has listed two key considerations raised by several research groups. First, “The effects of the large doses of antioxidants used in supplementation studies may be different from those of the smaller amounts of antioxidants consumed in foods”. Surely, anyone who has taken a multivitamin has noticed the absurd percent daily value (% DV) associated with just one serving. Next, “under some circumstances, free radicals actually may be beneficial rather than harmful, and removing them may be undesirable”. At first, this notion may seem contradictory to what you’ve been reading throughout this piece. However, despite the toxic effects of abnormally elevated ROS/RNS, basic science has shown us that within an appropriate physiological range, some of these molecules facilitate critical cellular functions. For example, the powerful oxidant hydrogen peroxide (H2/sub>O2, which you should not drink), has emerged as an important signaling molecule involved in cell division, growth, and inflammation. Interestingly, at low levels, H2O2 can stimulate the endogenous antioxidant system and actually help offset the harmful effects of stress. Thus, completely abolishing ROS/RNS through excessive antioxidant supplementation appears to be a poor strategy for improving health.
Reductive stress: An emerging phenomenon in human disease
Found on the opposite end of the redox spectrum, reductive stress is often characterized by a loss of physiological (healthy) ROS/RNS signaling and/or surplus of antioxidant molecules. Albeit far less studied than oxidative stress, reductive stress has recently been demonstrated in the development of neurological and cardiovascular disorders as well as cancer. Recently, those at risk for Alzheimer’s disease were shown to exhibit reductive stress, suggesting that antioxidant overabundance may serve as a useful diagnostic tool to help clinicians predict disease progression in these patients. In a heritable model of cardiomyopathy, high levels of antioxidant activity led to enlarged and dysfunctional hearts. In both the brain and heart, two highly oxidative tissues with minimal regenerative capacity, reductive stress is associated with the accumulation of toxic protein aggregates which presumably drives the eventual death of the cells in advanced stages of disease.
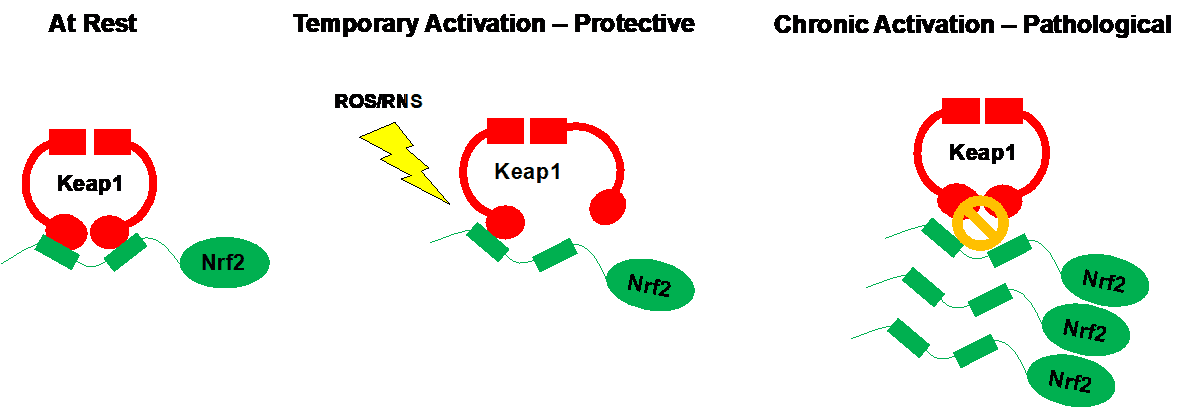
Unlike the non-dividing cells of the brain and heart, reductive stress apparently enhances the growth and survival of some particularly aggressive cancer cells. In response to chemotherapy, cells often reprogram their genes to enhance the expression of endogenous antioxidants in a feeble attempt to remove toxic byproducts of anticancer compounds. With successive drug administration, the goal is to eventually overwhelm and destroy these adaptive cells. While this works in some cases, several malignant cell types have developed ways to chronically boost their endogenous antioxidant defense system and become stubbornly resistant to chemotherapeutic agents. This can occur through the Keap1-Nrf2 signaling pathway.
Nrf2 is regarded as a master activator of endogenous antioxidant gene expression. In response to harmful ROS/RNS buildup, Nrf2 has the ability to promote the production of dozens of antioxidant proteins within the cell. However, in the absence of stress, the function of Nrf2 is inhibited by its binding partner Keap1. Cunningly, several tumor types have evolved to harbor DNA mutations in either Nrf2 or Keap1 such that endogenous antioxidant genes expression is always turned up to 11. Strikingly, in addition to appearing to accelerate cancer progression, four children suffering from undiagnosable disorders were recently found to possess Nrf2 mutations, in the genes controlling Nrf2, leading to its unrestricted activation. In these patients, sustained Nrf2 activity caused “an early onset multisystem disorder with failure to thrive, immunodeficiency and neurological symptoms”.
In spite of these reports, there are a number of laboratories persistently working to discover new pharmacological agents capable of enhancing Nrf2 activation. Sulforaphane, one such compound likely to be sold at your local supplements store, is currently being tested in clinical trials for prostate and lung cancer. While exogenous supplementation may be beneficial in some cases, the pathological evidence for oxidative and reductive stress suggest that additional research is needed to determine the optimal situation for antioxidant intervention. Indeed, careful consideration should be given when deciding on antioxidant regimens as these supplements are certainly not a guaranteed panacea for human health.
Take home message
By no means does this piece negate the decades of conclusive research illustrating the important protective role of antioxidants in cell biology. Rather, I hope this post helps convince you to be skeptical of the concept of antioxidants, and raise awareness of the under-appreciated reductive stress, in the field of biomedical research, and spread awareness to those regularly prescribing or consuming exogenous antioxidant supplements. Although many vitamins and minerals can be assessed by routine blood panels, I suspect that these values fluctuate considerably, and are not examined rigorously over time within most patients. Furthermore, the complex cross-talk among metabolic pathways makes it difficult to develop firm conclusions from any one single measurement. However, inferring redox status (e.g. oxidative vs reductive) from ratios of endogenous antioxidants such as glutathione may provide more useful diagnostic information. Hopefully, future research along these lines will look into stratifying patients according to their individual redox status, ideally leading to safer recommendations when it comes to antioxidant supplementation.

