The road from an idea to a useful drug is a long one, and in cancer it is often particularly long. One reason is that to be able to tell whether a given treatment is effective against cancer often takes several years at a minimum, in order to determine if patients receiving the new treatment are surviving their disease longer than those who are not. Surrogate endpoints are usually not enough. Tumor shrinkage in response to a drug often does not correlate with prolongation of survival, although the converse (i.e., lack of tumor shrinkage in response to a new drug) does strongly correlate with failure of a treatment to prolong survival. In other words, effects observed on surrogate endpoints are not enough to judge whether a cancer therapy is working or not.
Three years ago, predating the existence of this blog by nearly a year, I became aware of a story that involved many of the issues in bringing a compound from the laboratory to the clinic. The case was unusual in that is is very rare to see the scientific process by which new drugs progress through the stages of cancer research, from concept to testing in cell culture to testing in animals to testing in humans challenged so strongly by patients themselves. The reason that this normally doesn’t occur is that new cancer treatments are almost always the product of either university-conducted research, pharmaceutical company-conducted research, or partnerships between the two. This case was markedly different in that it involved a chemical that was not only easy to synthesize, but cheap and long out of patent. Even more intriguing, it targeted a metabolic abnormality found in many cancer cells, an abnormality first described nearly 80 years before by Otto Warburg in 1928. This latter aspect of the drug gave it every appearance of a “rediscovery” of old wisdom that big pharma had ignored for 80 years, and that only added to its mystique.
The chemical was dichloroacetate (DCA), and three years ago it created a world-wide sensation. Last week, it created a sensation again, as breathless news reports once again overhyped its promise. Since I’ve been following the story since early 2007, I appear to be in as good a position as anyone to tell the story thus far and put the new findings into context. To begin that process, let’s head back to January 2007.
“The cure for cancer big pharma doesn’t want you to know about”
The DCA saga began in January 2007, when I started noticing a bunch of posts by various bloggers as well as news stories that all had similar titles, such as Cheap, safe drug kills most cancers, Objectively pro-cancer, Gotta pay, When promising cures are ignored, and, my personal favorite, Potential cheap, safe cure for cancer: Will Big Pharma Allow It? These stories described some intriguing work by University of Alberta researcher Evangelos Michelakis regarding this old/new drug that showed great promise in cell culture and rodent models of cancer (more on that shortly) that was published in Cancer Cell. Typical quotes I saved at the time:
- “Big Pharma won’t put up the dough to fund human research and enable this drug to come to market, there’s no money in it. In fact, it wouldn’t surprise me to discover that they had an interest in actively preventing the research so as to maintain demand for more expensive less effective drugs. This drug looks to be extremely promising, and I can’t imagine that it won’t get government funding for human trials, but that said, it doesn’t pay to underestimate the power of Big Pharma…” (unfortunately the original link is gone, but the text lives on)
- “And here I thought the pharmaceutical companies had to charge such high prices because of all the research they were doing. Seems without the possibility of future revenue they can’t be bothered. Of course, a cheap cure for cancer would cut into profits in so many ways, wouldn’t it?” (From Digby, who really, really should have known better, but didn’t.)
- “It seems to good to be true. A cheap, effective cancer cure that BigPharma doesn’t own. If further research proves effective in humans, it could be the answer to many peoples prayers. I’ve always thought something simple, rather than the current convoluted regimen of surgery, radiation and chemicals would be the cure for cancer. Again, if proven effective, will we ever see it in use in this country? Will patients have to take ‘DCA tours’ to Canada for treatment?” (Source: Randular’s diary.)
- “Here’s the big catch. Pharmaceutical companies probably won’t invest in research into DCA because they won’t profit from it. It’s easy to make, unpatented and could be added to drinking water. Imagine, Gatorade with cancer control.” (Source; this particular editorial was truly overwrought.)
- “My bet is that the dichloroacetate news will fade into the background very quickly. Because neither the US government, nor any major university, nor any private company (pharma or otherwise) will have the least bit of interest in funding further studies. There will be a small handful of tiny, underfunded studies, and that will be that. Indeed, already those with vested financial and personal prestige issues will be doing their best to put the brakes on anyone who takes this development seriously. Not because the megacomplex of big government and big business wants to kill people–it doesn’t, there are few if any mustache-twirling villains–but because of the age old factors of bureaucracy, personal pettiness, ego, and conflicts of interest.” (Source: Dean Esmay, scroll down for post.)
Note that there were two assumptions here three years ago. First, these bloggers and pundits assumed that the cell culture and animal work were definitive evidence that DCA might be a “cure” for cancer. Second, the assumption was that, because the drug was out of patent and very cheap to make, neither the government nor pharmaceutical companies would be interested in funding it, thus condemning thousands, maybe millions, of people to die of cancer unnecessarily. Unfortunately, the New Scientist article and articles in the Edmonton Sun featured headlines to that effect and quotes by the investigator Evangelos Michelakis lamenting how he had had difficulties finding funding to do the next step, clinical trials in cancer. As a result of these sensationalistic stories, unscrupulous “businessmen” sought to bring DCA to the masses.
DCA and the Warburg effect
DCA is a very simple molecule, deceptively simple. Basically, it is an analog of acetic acid in which two of the three hydrogen atoms of the methyl group have been replaced by chlorine atoms. Interestingly, it is only one chlorine atom off from trichloroacetic acid (TCA), a chemical we routinely use in the laboratory to precipitate nucleic acids and proteins from solution. The structure of DCA is depicted below.
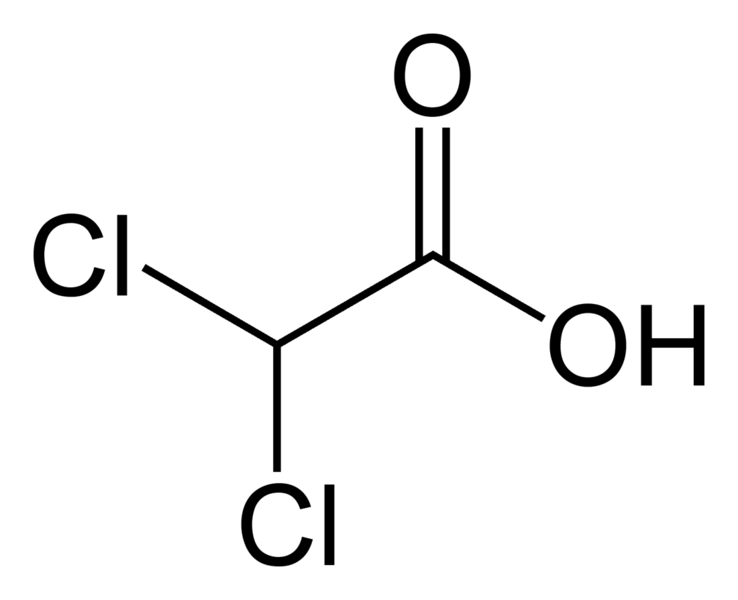
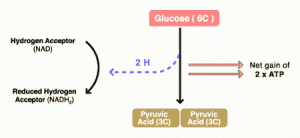
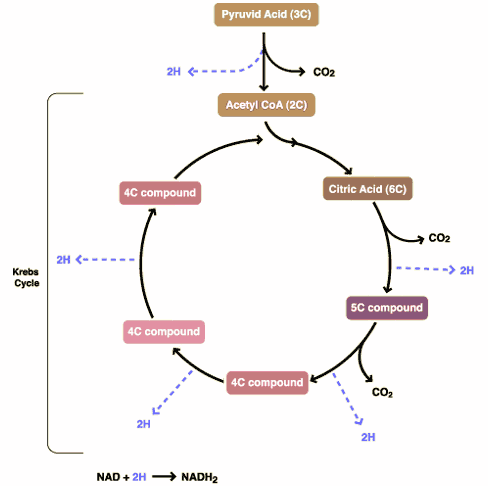
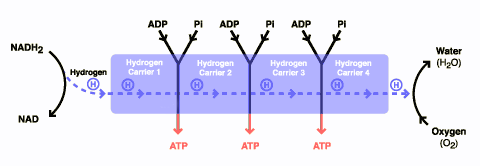
DCA has been around for a long time (which is why is it no longer under patent) and has primarily been used for inherited diseases of mitochondrial metabolism. Mitochondria are often (and correctly) referred to as the “powerhouses” or the “batteries” of the cell, because it is in the mitochondria that the main energy-containing molecule, ATP, is produced using byproducts of glycolysis and the Krebs citric acid cycle to generate a proton gradient across the mitochondrial membrane, which supplies the energy for the enzyme ATP synthase, which, true to its name, synthesizes ATP for use in the cell as a chemical source of energy. The key thing to remember about oxidative phophorylation is that it requires oxygen, whereas glycolysis does not. When there is insufficient oxygen, the end products of glycolysis end up being turned into lactic acid, which is one of the things that make muscles feel tired after a rapid workout that exceeds the capacity of the body to deliver oxygen to the tissues. The primary activity of DCA in cells is to inhibit the enzyme pyruvate dehydrogenase kinase. The result, to boil it down (and not to have to stress my knowledge of the basic biochemistry of glycolosis, the Krebs cycle, and oxidative phosphorylation too much) is to shift the metabolism of pyruvate from glycolysis towards oxidation in the mitochondria. To boil it down even further, DCA shifts the cell’s metabolism from anaerobic to aerobic metabolism.
The three components of glucose metabolism are shown below in simplified illustrations:
Why, then, would such an activity be useful as an anticancer therapy?
It all boils down to something known as the Warburg effect, which Otto Warburg first described way back in 1928 and reported in Science back in 1956. Over the last five years or so, cancer researchers have been increasingly coming to appreciate the role of abnormalities in metabolism, in particular the mitochondria, in cancer. To put it briefly, many cancers (approximately 60-90%) favor glycolysis, even in the presence of adequate oxygen for oxidative phosphorylation, leading to a voracious appetite for glucose. Indeed, it is this very avidity of cancer cells for glucose that is the basis of the PET scan, which detects the high uptake of a radiolabeled form of glucose by cancer cells relative to the surrounding normal cells.
Over the last few years, there has been a sort of “chicken or the egg” argument about what is more important and what is the first abnormality leading to cancer. The traditional view has long been that mutations in DNA lead to the activation of protooncogenes into cancer-initiating and causing oncogenes and to the shutdown of tumor suppressor genes. Under this model, mutations leading to cancer also lead to the observations of abnormalities in metabolism. In the wake of the DCA furor, there have been data reported suggesting that the metabolic derangements may actually occur first or simultaneously with the mutations. p53, for instance, the granddaddy of tumor suppressor genes, can trigger the Warburg effect when mutated. Whatever the case, it is now fairly clear that abnormalities in cancer cell metabolism are very important in driving cancer growth and could well represent targets for cancer therapy. AS a result of these new data, studying the metabolism of cancer cells has become a much hotter topic of research than it has been in the past. Everything old is new again, it seems. Why cancer cells might have an advantage due to the Warburg effect is a matter of debate, although, given how tumors frequently outgrow their blood supply, being able to maintain themselves in low oxygen situations would be advantageous.
This fascinating basic science met the public in January 2007, when Michelakis and his colleagues at the University of Alberta in Edmonton published a seminal paper in Cancer Cell. In the study, DCA was tested in multiple cell culture and rodent models of cancer. In rats, tumor weights in animals treated with DCA were approximately 60% lower than the tumors in the untreated control groups. The drug increased apoptosis, decreased proliferation, and inhibits tumor growth by acting on a critical enzyme that controls the switch between aerobic and anaerobic metabolism without harming non-cancerous cells. Even better, DCA had already been FDA-approved for mitochondrial disorders, meaning that using it in humans would be an “off-label” use of an already existing drug to test it in humans. Thus, the regulatory requirements were considerably easier to meet for early drug trials in cancer.
The “Pet DCA” phenomenon
The media coverage of the Michelakis study unleashed the proverbial perfect storm of conspiracy mongering about big pharma. Never before (at least that I can recall) had a chemical that was so cheap and so easy to synthesize, not to mention already used in patients for another indication, been reported as being a “cure” for cancer. This hype, of course, neglected what those of us who do cancer research as part of our living, namely that many are the candidate drugs that show a great deal of promise in animals but fail in humans and that at present there is no such thing as a “cure for cancer.” I like to think of the example of angiogenesis inhibitors, which I’ve been studying for 13 years now. Part of what attracted me to the study of these compounds were the amazing results of the late Dr. Judah Folkman in mice, which I’ve described on this blog before. However, the amazing results in mice did not translate to humans. That’s not to say that angiogenesis inhibitors don’t work, but in humans they did not produce anywhere near as dramatic results as they did in Dr. Folkman’s mouse experiments. That’s why, rather than being a “magic bullet” for cancer, angiogenesis inhibitors entered our armamentarium of anti-cancer drugs as a useful, but not miraculous, new addition. Avastin, for instance, has increased the five year survival rates in colorectal cancer metatastic to the liver and several other cancers, when added to the current standard of care, but it has not resulted in the cure of any metastatic cancer.
That being said, I can understand how cancer patients incurable with standard therapy would leap at this drug as their last hope. Unfortunately, there were unethical “entrepreneurs” who were more than eager to supply DCA to them. The most famous of these who appeared in the months following the publicity surrounding Michelakis’ study was a pesticide dealer named Jim Tassano, who leapt to create a website known as TheDCASite.com and BuyDCA.com (the latter of which appears to be unreachable at the moment). His way of getting around the FDA? To market his DCA as “Pet DCA,” to be sold for pets dying of cancer. It was as disingenuous an approach as could be imagined, because not only did Tassano openly admit that he knew that people were buying the drug for themselves but he blithely dismissed the possibility of serious side effects in adults. Although children could take the drug with few side effects, adults who took the drug often developed serious severe peripheral neuropathy. Since peripheral neuropathy is a side effect of some commonly used cancer chemotherapeutics, taking DCA with these cancer chemotherapies has the potential to do serious harm. Moreover, when asked about the source of DCA, the webmaster Heather Nordstrom replied:
In my opinion, it is not that difficult to get, but that may be because my step father has connections with manufacturers since we invent and sell tools for our family business. We know of a chemical company in China that makes DCA. It is pharmaceutical grade. More information on the quality and source will be posted on the website that sells it.
Naturally, I was…skeptical. Worse, scammers were hyping DCA as not being “chemotherapy,” when, if it works for cancer, that’s exactly what it is.
Meanwhile, cancer patients and their families flooded the discussion boards of TheDCASite.com to write about their self-experimentation. Here are a couple of posts that I saved (the DCASite.com purged many of its forums when it started to get into legal trouble):
My husband has been using DCA since early February. He has Glioblastoma, an aggressive brain cancer that DCA is proposed to target. The naDCA he is using was made in a private lab. We turned away from our medical community, realizing that we would not receive blessings from them, since they considered him a “dead man walking”. From what I have read here, my husband seems to be the earliest “labrat”. We obtained the DCA in early February, started at a 5% dose,(to test toxicity or side effects, I suppose) and after 4 days , he insisted on taking 25mg per kg. He takes a liquid dose twice a day( totaling 25mg per kg). He has been taking DCA since Feb 7, 2007, with full dose as of Feb 11. No side effects to report as yet. Though side effects of DCA(numbness in fingers and appendages) are also symptoms of his disease, there are none to report at this time. He is also taking 100mg per day of thiamine. He is also on CCNU, Heparin, and 16 mg steroid. So far, so good. I am taking weekly urine samples to check his billyrubin, ph , etc. He still sees an oncologist, and takes chemo( CCNU). His doctors do not know that he is taking DCA. I do not trust them, they have not been terribly compassionate through this, and I do not feel that they would be as knowledgable as those of us that are in these desparate situations. I pray for all of us that I can report great news in the weeks to come. I still can’t decide at this point if he should take THiamine or not? Any thoughts?
My thought at the time was that this woman’s husband was endangering his life by taking DCA along with his chemotherapy and not telling his doctors about it. Meanwhile, as time went on, someone on the DCASite actually questioned the anecdotes in May 2007:
Maybe I am crazy but it seems like every time I ask this question the admin removes it – if not then where is the question I asked last time?
I am asking this Question –
Do we want to know or not? is this a site for lies or truth? I have DCA and I also sent money in for the clinical trials as well as having my wife dealing with stage four lung cancer currently stable with antioxident regimens. Today we go in with DCA to see if her oncologist will support her using it.
Some news here would sure be great but NO ONE who has been CT scanned after using DCA for a couple months is talking or coming back except for squareb who gave us bad news. What is going on here? are we participating in a venting session where these folks are linked with those folks selling us DCA? what exactly is going on – our lives and our loved ones lives are on the line and not ONE CT SCAN in 4 months!!!! or the TRUTH is being removed from the site and people who had scans did post bad results so it was also removed like my questions are.
I am not happy about this admin – gee sorry I lost everything sorry – what? didn’t this happen last month on another persons testimony who DIED!! while using DCA. Something is definitely missing here and I am starting to think its THE TRUTH.
I believe in DCA but I am starting to think these admins are manipulating the information to suit them which is pretty typical for us trying to survive to run into. Sorry but these admins need to either post it ALL or explain why they are not allowing CT scan results to be listed on this site. Folks just think about it – Jan, Feb, March, April and now May – 4 months and nothing – come on folks lets get real here – do you really think its a coincidence?.
It is too bad that more people didn’t ask questions like this. squareb, by the way, was an early adopter of DCA whose tumor progressed on the drug.
Unfortunately, Jim Tassano wasn’t alone. For example, a family physician in Toronto named Dr. Humaira Khan, even though agreeing with the warnings not to use DCA outside the auspices of a clinical trial, decided to start selling DCA for $150 a week, using arguments of “health freedom” and wanting to “supervise” patients who would take the drug anyway. Dr. Khan’s webpage on DCA appears not to have been updated since 2008 but is claiming a high response rate. Odd, though, I haven’t seen this published anywhere in the peer-reviewed scientific literature, and a PubMed search turns up…nothing, at least nothing by Dr. Khan or anyone associated with her. Jim Tassano himself also tried to do a “clinical trial” of DCA that was basically nothing more than a questionnaire that–I kid you not–included questions like:
- Do you think DCA is working for you? Why or why not? Scans? Examinations? Blood level testing? Here, I encourage you to freeform answer.
- How do you rate your health compared to when you started DCA? 1. Much better; 2. Somewhat better; 3. About the same; 4. Somewhat worse; 5. Much worse
I trust that SBM readers will immediately recognize why such questions are worse than useless. Basically, they are doing nothing more than soliciting testimonials, and testimonials in cancer are often highly misleading, and that’s all TheDCASite has now. This is just as true for something like DCA as it is for any alternative medicine cancer therapies. Fortunately, in July 2007 the FDA finally acted to shut down TheDCASite.com, at least as far as selling DCA went. In response, Tassano shut down BuyDCA.com and morphed TheDCASite.com into an “informational” website. Much of what was on the forums there disappeared. Meanwhile, conspiracy theorists had a field day denouncing the FDA’s action.
Back to the future
Over the last couple of years, my biggest fear was that the activities of “entrepreneurs” like Jim Tassano would taint what is a scientifically fascinating and potentially very useful new cancer therapy with the indelible stain of quackery. DCA is most definitely not quackery. It is, however, unproven in humans and thus may not be effective, which is why self-treatment and treatment by doctors who have no clue what they are doing are not advisable. Fortunately, while ignorant businessmen like Tassano and opportunistic doctors were selling DCA to desperate cancer patients, a clinical trial was beginning by Michelakis and his team, and last week the results were reported in Science Translational Medicine entitled Metabolic Modulation of Glioblastoma with Dichloroacetate.
The first part of the study was something quite fascinating that I don’t recall ever having seen before in a clinical trial. Michelakis and colleagues studied 49 consecutive surgically excised glioblastomas. Glioblastoma is an aggressive form of brain cancer known to exhibit the Warburg effect and thus a good candidate for the first attempts at testing DCA in humans. These tumors were then tested in vitro with DCA. The excised tumors did demonstrate evidence of the Warburg effect, and treatment with DCA resulted in significant reversal of some of these features in the excised tumors, particularly mitochondrial hyperpolarization, while not having any effect on normal tissues excised with the cancers. The second part of the study is what most media and blog reports focused on. Michelakis treated five patients with neuroblastoma with oral DCA. However–and this needs to be emphasized–patients were also treated with temozolomide and radiation, with DCA added to the mix. The DCA dose started at 12.5 mg/kg orally twice a day for 1 month, and then the dose was increased to 25mg/kg orally twice a day. Michelakis then followed a dose de-escalation protocol, decreasing the dose by 50% when dose-limiting toxicity occurred. The patients were followed clinically for up to 15 months. In other words, this appeared to be a combined phase 0/phase I clinical trial.
For those not familiar with the various types of clinical trials, phase I clinical trials are not trials of efficacy. They are designed to determine two things: dose and dose-limiting side effects. They generally use a few patients (although five patients represent a rather small number, even for a phase I trial, which usually requires around 10 or 20), and it is not uncommon to perform a dose escalation. Researchers don’t expect necessarily to see tumor response in a phase I trial, as that is not the purpose of the trial, but it is heartening when tumor shrinkage is observed, for obvious reasons. Phase 0 trials similarly are not therapeutic trials but rather seek to determine if the drug is doing biochemically what it is expected to do based on preclinical studies. The usual design is to take a biopsy of the tumor, test it for biochemical markers in the laboratory, treat the patient with experimental drug, and then resect the tumor. The biochemical markers in the resected tumor are then compared with those measured in the pre-treatment biopsy. The idea is to see whether the drug can recapitulate biochemical changes in actual living tumors in human patients, the idea being that, if it can, then the drug is “hitting the target” (i.e., its molecular target) and therefore “working.” Whether its “working” actually shrinks tumors or results in prolonged patient survival is then the next question that has to be tested.
Michelakis went one further in that he isolated tumor cells from the pretreatment biopsies and produced glioblastoma cell lines. He could do this in three of the patients because he had tissue from their first debulking surgery, and these patients had recurrent glioblastoma that had failed additional chemotherapy (patients 1 through 3). All of them had had multiple rounds of different chemotherapy. Patients #1 and #2 showed signs of improvement or were at least stable for the full 15 months of the study, while patient #3 died three months after starting DCA therapy. Two other patients (patients #4 and #5) had new diagnoses, allowing initial tumor tissue from debulking surgery to be examined. Their treatment was somewhat different. After his first surgery, patient #4 underwent DCA therapy for three months followed by DCA and standard therapy. However, by the end of the third month, the patient showed evidence of progression requiring a second operation. Patient #5 underwent surgery followed by DCA and radiation with temozolomide for six months and then stayed on DCA for nine more months and is doing well.
The reason I mention this is because not only were there only five patients, but they were not all even treated the same way. They were treated with varying regimens of surgery, with drug therapies combining DCA and chemotherapy (mostly temozolomide), some with and some without radiation. Even so, the tissue from these tumors showed signs of metabolic reversion to an oxidative phosphorylation phenotype. The problem is, as both our very own David Kroll pointed out, we don’t know for sure if the DCA was responsible for this effect. As a cancer researcher, I can’t say whether the regressions observed were due to DCA, although the regression of paraventricular masses in patient #1 and the regression observed in patient #5 are certainly fairly suggestive (at least to me) of an anti-tumor effect due to DCA alone. The only side effect Michelakis reported was a reversible change in peripheral nerve function.
So what does this all mean?
I’m frequently asked why we shouldn’t just use DCA now–or even let people use it the way that they were using Jim Tassano’s homemade DCA? What’s the harm? That’s a rather difficult question, because there is always a conflict between wanting to do something now for suffering patients, damn the consequences, and following the scientific method to demonstrate efficacy and safety. Our nation has been at both extremes. Indeed, until 1906, pharmaceutical companies could make essentially any claims and sell essentially anything to the public as a drug without regulation. We all know how well that worked out. Early in the history of the FDA, as Dr Jerome Groopman points out, companies often tested new drugs by sending them to doctors to offer to their patients, asked for little information regarding side effects and complications, and had no standard criteria for efficacy. There was a reason we moved away from such a system.
I think Dr. J. Leonard Lichtenfeld, Deputy Chief Medical Officer for the national office of the American Cancer Society, put it well in writing about this latest DCA study on his blog, Dr. Len’s Cancer Blog:
This research still needs lots of work before we know whether it works or doesn’t work, and whether it is really safe or not when given to patients with cancer under a variety of circumstances.
If that sounds overly cautious, so be it. I have seen too many dashed hopes in my medical career which make me a bit cautious about reports like this. That’s not to say I don’t think it could work—it could, as I mentioned above—but I want to see evidence in well done trials that prove the point that DCA is effective in the treatment of which cancers under what circumstances.
Early in my cancer training there was a substance isolated by a researcher that was supposedly non-toxic and would cure leukemia. The research center where I was working was inundated from people around the globe who wanted this treatment, especially after the lead researcher injected himself on a nationwide morning show to demonstrate its apparent lack of toxicity.
Only grams of this medicine existed. Fortunes were offered in return for getting this miracle drug.
But the miracle drug—after reasonable clinical trials were done—didn’t work after all.
Many are the lists of new “miracle cures” that have met this same fate. The difference today is that the Internet has allowed news of these drugs to be disseminated to more people than ever before–and faster than every before. Moreover, it has linked patients and activists into mutually supportive disease-specific communities, who can inform and educate each other, as well as publicizing research about their disease and lobbying legislators. The dark side of this power, however, is that it can facilitate the spread of false hope and the demand for a drug after only cell culture and animal work, before it even makes it to human trials. Add unscrupulous “entrepreneurs” into the mix, and the potential for harm is great.
One has to remember that cancer is not just one disease. Not only that, but even a single type cancer is often not just one disease. As I have written extensively about before, cancer is incredibly complex. Because of that complexity, it’s incredibly unlikely that any one drug will be any sort of “magic bullet” to cure cancer. Worse, simply using a drug like DCA outside the auspices of well-designed clinical trials will virtually guarantee that we will never know for sure whether the drug actually works. Because of that, as frustrating as it is, as slow as it is, letting science take its course to determine if DCA works, how it works, and for what cancers it works, is the best method to make sure that the most patients are helped and the fewest are harmed. I don’t say this because I want DCA to fail; I say it because I would very much like to see DCA succeed.
Other good posts about DCA: