If there’s one thing I’ve been consistent about, it’s that, however ridiculous all the other woo I routinely discuss here is—homeopathy, reiki, reflexology, I’m talking to you and your friends—herbal medicine and supplements might have value because they might have a physiological effect that is beneficial in treating or preventing disease. Of course, if that’s the case, it’s because the herb or supplement contains chemicals that act as drugs. They’re “dirty” drugs in that they are mixed with all sorts of other substances in the herb or supplement that might or might not have effects, which means that different lots of the herbs or supplements often have different activity, but they are drugs nonetheless. That’s why, for instance, doctors don’t tell patients to chew on foxglove leaves when they want a patient to get digoxin. Digoxin is a powerful drug with a relatively narrow “therapeutic window,” meaning that the difference between the levels of the drug in the blood needed for therapeutic effect are not very far from toxic levels; so predictable, reliable drug content is essential. I just learned a while ago that within the living memory of some older physicians digoxin actually was prescribed as crude extracts, which was very difficult and dangerous, hence the necessity of purification. In other cases, (such as Artemisinin, for which Youyou Tu was recently awarded the Nobel Prize in Physiology or Medicine), crude plant extracts do not contain sufficient quantities of the active component, necessitating its isolation, purification, and, in some cases, chemical modification to increase its absorption, stability, or activity.
One thing that proponents of herbal medicine and supplements often forget, though, is that if herbs or supplements can have potentially beneficial effects (albeit difficult to regulate effects due to the crude, impure nature of the extracts often used) because they contain drugs, then herbs and supplements can also produce adverse events, again, because they contain drugs. You can overdose on herbs and supplements. This point was recently reinforced by a new study by Geller et al. published last week in the New England Journal of Medicine (NEJM), entitled “Emergency Department Visits for Adverse Events Related to Dietary Supplements.” It was carried out by investigators from the Division of Healthcare Quality Promotion, Centers for Disease Control and Prevention, and Chenega Government Consulting; and the Center for Food Safety and Applied Nutrition and the Division of Public Health Informatics and Analytics and the Division of Dietary Supplement Programs, Food and Drug Administration. The title pretty much tells you what the study is about, and what the study is about is that dietary supplements cause a lot of visits to the emergency room every year; 23,005 (95% confidence interval [CI], 18,611 to 27,398) emergency department visits per year can be attributed to adverse events related to dietary supplements.
Let’s back it up a minute before I get into the study more. In the US, dietary supplements are regulated by the FDA (or, more appropriately, barely regulated by the FDA) under the Dietary Supplement Health and Education Act of 1994 (DSHEA), which is one of the worst laws ever passed or, as Peter Lipson called it right here, a travesty of a mockery of a sham. Basically, supplements are considered more food than medicine and regulated as such. They can’t be marketed for the treatment or prevention of disease (i.e. drug claims), but can make so-called “structure-function” claims (i.e., “boosts the immune system”). The problem is, what constitutes a “drug claim” compared to a “structure-function” claim remains fairly vague. Even after the FDA issued rules in 2000 that banned explicit claims that a product treats or prevents disease, there’s still considerable wiggle room, and enforcement isn’t exactly what I would call robust. In the meantime, supplement hawkers have cleverly used the Internet to circulate claims for these products that aren’t necessarily on the packages or in the package inserts. As Geller et al. note:
Although supplements cannot be marketed for the treatment or prevention of disease, they are often taken to address symptoms or illnesses, as well as to maintain or improve overall health. The estimated number of supplement products increased from 4000 in 19943 to more than 55,000 in 2012 (the most recent year for which data are publicly available), and approximately half of all adults in the United States report having used at least one dietary supplement in the past month.5 In 2007, out-of-pocket expenditures for herbal or complementary nutritional products reached $14.8 billion, one third of the out-of-pocket expenditures for prescription drugs.6
We know supplements are big industry here in the US. Indeed, for whatever reason, Utah has become the state where the supplement industry is most concentrated, and as a result its politicians strongly support supplements. Senator Orrin Hatch (R-UT) has long been in the pocket of the supplement industry and is its most friendly lapdog and powerful defender. Rising star Representative Jason Chaffetz (R-UT) is a long shot candidate for Speaker of the House in the wake of John Boehner’s resignation and the implosion of Kevin McCarthy’s (R-CA) candidacy, and Chaffetz has over the last few years been vying to outdo Hatch in demonstrating his fealty to the supplement industry. It’s not just Utah politicians, though. There is the Congressional Dietary Supplement Caucus, which is bipartisan and has members from all over the country. It includes the aforementioned Chaffetz and Hatch, as well as Sen. Darrell Issa (R-CA), Rep. Frank Pallone (D-NJ), and Rep. Tom Cotton (R-AR). Thanks to the power of the supplement industry, thus far every attempt to tighten regulation of supplements since 1994 has failed. For example, in 2010, facing a primary challenge from the Tea Party, after having championed a bill to give the FDA more power to regulate dietary supplements, Sen. John McCain (R-AZ) caved because he decided that he couldn’t be perceived as being in favor of more government regulation.
As a result of the lack of regulation of dietary supplements, manufacturers and advocates make all sorts of de facto health claims for supplements such as that they can prevent or treat cancer and the like. FDA enforcement is sporadic and directed at only the most egregious, most immediately dangerous cases. Meanwhile, advocates, using the exaggeration of “food as medicine,” continue to sell their products, and many supplements are scary substances manufactured under scary conditions, even though there is a distinct paucity of evidence for their usefulness.
The result is what we see in this study. Postmarketing reporting of adverse events from supplements by manufacturers is only required for the most serious events (e.g., those resulting in hospitalization, significant disability, or death), while voluntary reporting almost certainly greatly underestimates the incidence of adverse events. So Geller et al. decided to use a national surveillance database to estimate the number of adverse events attributable to supplements that result in visits to the emergency room. They used the National Electronic Injury Surveillance System-Cooperative Adverse Drug Event Surveillance (NEISS-CADES) project conducted by the CDC, the FDA, and the Consumer Product Safety Commission. There are 63 hospitals participating in the project that make up a nationally-representative probability sample drawn from all hospitals with at least six beds and 24-hour emergency departments with four strata based on hospital size and a fifth stratum for pediatric hospitals. Cases were identified thusly:
Trained abstractors at each hospital reviewed the clinical records of every emergency department visit to identify physician-diagnosed adverse events and reported up to 2 implicated products and 10 concomitant products, as described previously. Abstractors also recorded narrative descriptions of the event, including preceding circumstances, physician diagnoses, testing, treatments administered in the emergency department or by emergency medical services, and patient outcome. Narrative data were coded with the use of the Medical Dictionary for Regulatory Activities (MedDRA), version 9.1.
Geller et al. looked at a ten year period from 2004 to 2013 for cases:
Cases were defined as emergency department visits for problems that the treating clinician explicitly attributed to the use of dietary supplements. This analysis included orally administered herbal or complementary nutritional products (including botanicals, microbial additives, and amino acids) and micronutrients (vitamins and minerals) but excluded products that are typically considered to be foods or drinks (e.g., energy drinks, herbal tea beverages). Additional products that are often used by consumers for complementary health but do not fall under the regulatory definition of dietary supplements (e.g., topically administered herbal or homeopathic products) were also included in the analysis. Herbal or complementary nutritional products were categorized according to common reasons for use…
Adverse events were categorized as adverse reactions, allergic reactions, excess doses, unsupervised ingestion by children, or other events (e.g., choking). Cases involving death on the way to the emergency department or after arrival were excluded because death registration practices vary among participating hospitals, and details about event circumstances are often lacking. Visits involving intentional self-harm, drug abuse, therapeutic failures, nonadherence, or withdrawal were also excluded. The categorization of symptoms was based on MedDRA-coded narratives.
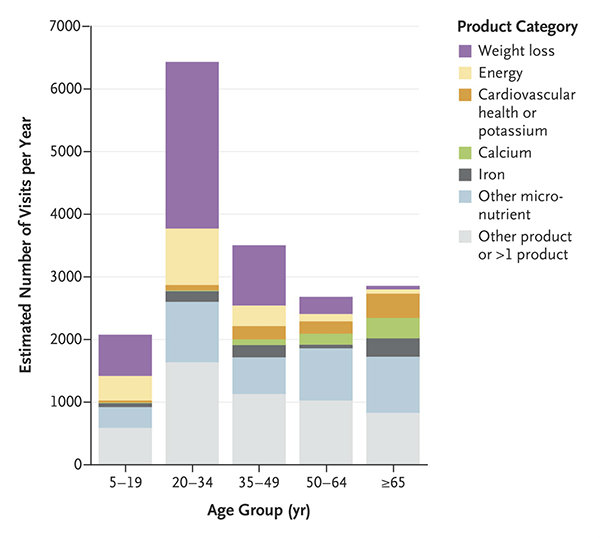
When the analysis was done, on the basis of 3.667 cases identified in the database, Geller et al. calculated an average of 23,005 emergency room visits for adverse events related to supplements and further estimated a 2,154 yearly hospitalizations. In the vast majority of these cases (88.3%), doctors attributed the adverse event to only one supplement, and the mean age of patients was 32 years, with more than a quarter of the visits due to patients between 20 and 34 years of age. Not surprisingly, people older than 65 were nearly twice as likely to be hospitalized. One-fifth of the visits involved unsupervised ingestion of supplements by children. (So, parents, lock your supplements up the way you do with your prescription and over-the-counter drugs.) There’s a nice graph in the article that boils down a lot of information in a very compact package, including age of patients and what the product category was:
Not surprisingly, in younger patients weight loss supplements were by far the largest category of supplements that caused adverse events. Similarly, not surprisingly, cardiac symptoms were the most common symptom associated with weight-loss products (42.9% of patients), and weight-loss or energy products were implicated in 71.8% of all ER visits for supplement-related adverse events involving palpitations, chest pain, or tachycardia. These were young people, too, the vast majority between 20 and 34 years old. Interestingly, among patients under 6 and 65 or older, swallowing problems caused a significant number of the ER visits. I don’t find that too surprising, given the horse pill-sized capsules many supplements are often sold in. Calcium supplements were the worst offenders.
It’s rather an odd coincidence that a couple of days before this study was published, the story of NBA star Lamar Odom’s fight for his life after taking large quantities of “herbal Viagra” in a Nevada brothel hit the news in a big way. It should be noted, however, that Odom had also apparently been using cocaine and drinking Cognac, so it’s difficult to know what the cause of his collapse was for sure, although it certainly could have been a combination of whatever was in the herbal product plus the cocaine, plus whatever else he was taking (and, according to E! News, he was taking a lot). Whatever the case, Odom is, by many reports, in bad shape, with TMZ.com reporting the failure of four organs, is on dialysis, and has suffered multiple small strokes. How many of these medical reports are true (considering the source) or not, the prognosis of multiorgan failure is very, very bad.
Whether the ten or more tablets of “herbal Viagra” that Odom took had anything to do with his condition or it was the cocaine, Cognac, and other drugs he was doing that landed him hear death, this NEJM study is yet more evidence that there are real consequences to the DSHEA and letting supplements be sold with, in essence, almost no regulation. Odom, if the herbs were the main reason for his collapse, would just be an extreme case.