Osteoarthritis (OA) is a chronic joint condition that can include pain, stiffness, and limitations on a joint’s range of motion. It commonly affects the knees but can also be found in other joints in the body. The traditional view of osteoarthritis was that it was a consequence of wear and tear on the joint, leading to cartilage thinning and ultimately, bone-on-bone friction. The reality of OA is more complex: What appears in investigations (like X-rays) does not reliably correlate with pain and other symptoms. Osteoarthritis is increasingly considered a whole body or systemic condition with a variety of contributing factors, which can ultimately manifest as joint pain. It is the old, narrow view of OA that led to the traditional perception that joints were “worn out” and that rest and drugs were preferred over more active therapies. This was misguided and wrong.
Physical activity and active management are now the preferred primary treatments for OA. However there continues to be a heavy reliance on medications to treat OA. These treatments range from simple analgesics (like acetaminophen (e.g., Tylenol)), anti-inflammatory drugs (e.g., ibuprofen or naproxen), topical treatments (as I have written about before), and less commonly, opioids, injections, and supplements like glucosamine and chondroitin. For the treatment of OA, there is good evidence that most of these treatments provide minor/modest benefits compared to placebos. Moreover, many of these products have substantial side effect profiles that make them undesirable for use for a chronic condition, like OA.
A preferred new treatment for OA would provide substantial pain relief compared to a placebo, with an acceptable side effect profile. It would ideally target some of the underlying pain signalling pathways that have been observed. Turmeric (Curcuma longa) has a long history of use as a traditional medicine and as a food ingredient. The main ingredient, curcumin, has a number of interesting biological properties, and it has been studied as a treatment for OA before. A 2017 systematic review compiled all the evidence and concluded that curcumin was an effective therapy, albeit less effective than ibuprofen. Even that conclusion was weak, given the diversity and overal poor quality of the original studies, all of which had been done in Asia. This brings us to a new randomized controlled trial that examined the effectiveness of curcumin on OA in adults in Tasmania.
The trial
Before we get into the trial, some definitions:
- Curcuma longa is the plant (in the ginger family).
- Turmeric is the product of the root of the Curcuma longa plant, after it is dried and ground up. Turmeric is the spice that you buy.
- Curcumin is one of the chemicals in turmeric, and thought to be one of the ingredients that has medicinal benefits and effects. It is one of a family of curcuminoids in turmeric.
The main challenge with using curcumin as a medicine seems to be that the chemical is poorly absorbed. Manufacturers have come up with several different formulations (e.g., nanoparticles, liposomes) which complicates how we interpret the evidence. Given how poorly it absorbed, it would be a stretch to assume that a positive trial with one product can be mimicked with another formulation or brand of curcumin. That will be important to keep in mind when considering this new trial.
This new trial is entitled “Effectiveness of Curcuma longa Extract for the Treatment of Symptoms and Effusion–Synovitis of Knee Osteoarthritis” and is from Zhiqiang Wang and colleagues at the University of Tasmania. It was a randomized, double-blind, and placebo controlled trial which enrolled 70 patients with symptoms of knee osteoarthritis, and signs of osteoarthritis observable with MRI. Participants had to be over the age of 40 and have a pain score of at least 4/10 (or 40/100) on a visual analogue scale:

A visual analog pain scale
One of the challenges in measuring pain, especially in clinical trials, is that there’s no objective marker. We rely primarily on visual analogue scales (0-100, or 0-10) and asking people to rate their pain (“How does your pain feel on a scale of 0 to 10”). The image above is an example of a scale that might be used. An important question when using a pain scale is determining what constitutes a “meaningful” difference. This often comes up in the discussion of alternative medicine where a very weak (but statistically significant) difference is shown in some measure, which is cited as “proof” that the intervention (e.g., acupuncture) “works”. We use the term “clinically important” or “clinically relevant” to try to distinguish between changes that are meaningful to patients, and those that are likely not. For example, if you rate your pain a 70 today and a 66 tomorrow, that’s not likely to be a clinically meaningful difference.
Back to the trial: patients were randomly assigned to take a standardized formulation of curcumin (Tumacin Plus) (1000mg/day) or an inert placebo. The dose was based in part on other randomized controlled trials of curcumin.
The two outcomes of interest were pain, as measured on the visual analogue scale, and changes in knee effusion-synovitis as assessed by MRI. They also looked at knee pain and function, medication use, quality of life, and adverse effects reported. Any medications that were being taken could be continued, but participants were asked to keep their medication use as stable as possible.
The results
Over a twelve-week period, knee pain improved more in the curcumin group (-23.8mm on the scale) compared to the placebo group (-14.6mm on the scale) resulting in a difference of -9.1mm that was statistically significant. (Whether a difference of just under 10mm is meaningful to a patient is more subjective.) There were no differences between the groups as detected by MRI. Here are all the findings for all of the measures:
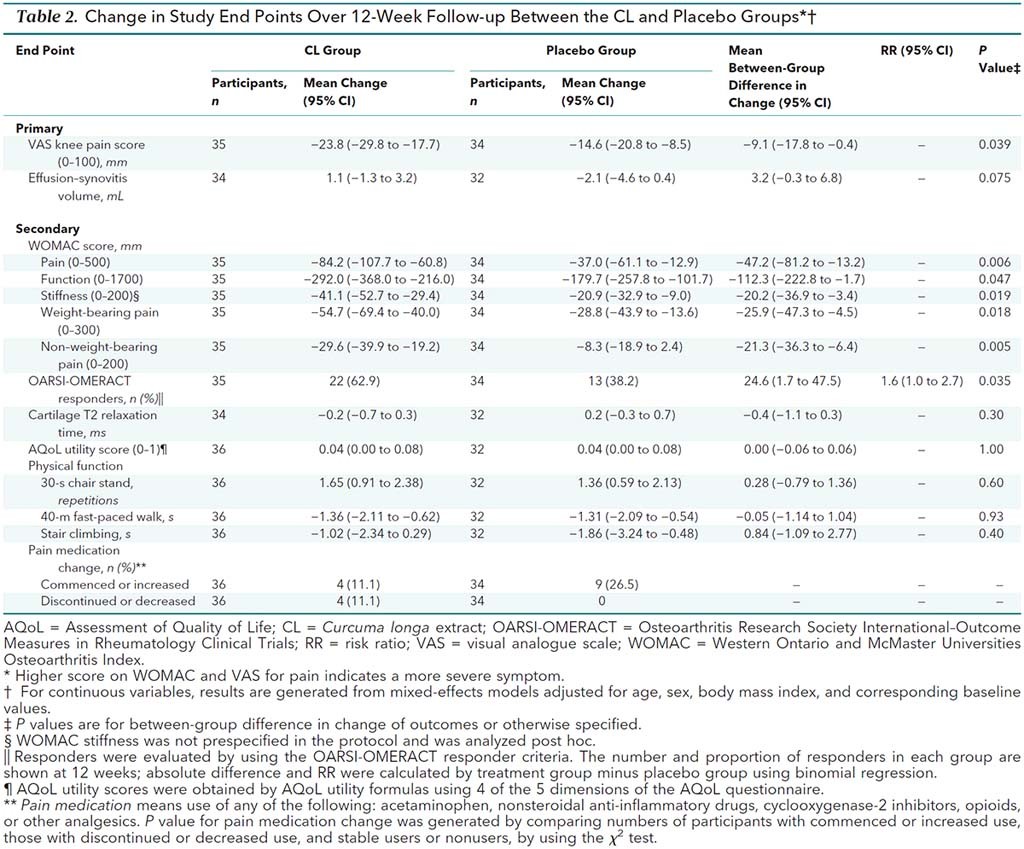
As you can see, several of the secondary measures were statistically significant as well. Interestingly, four patients in the curcumin group discontinued or decreased their medication use during the trial, compared with none in the placebo group.
On balance it looks like curcumin was better tolerated than the placebo. 39% of the curcumin group reported at least one adverse event, compared to 53% in the placebo group.
Conclusion: Promising but modest effects
Compared to placebo, this trial showed curcumin modestly reduced knee pain over 12 weeks, with no effects observed based on MRI. The treatment was well tolerated as well. The authors comment that the clinical significance of this finding is unclear. However they correctly point out that other analgesics like acetaminophen are equally unimpressive in terms of reported pain relief, and arguably have a much less favourable side effect profile. There were no objective effects on knee joint swelling, suggesting that the effects may be due to perceptions of pain that are not related to the manifestations of the disease in the joint.
Turmeric supplements have promise as a treatment for osteoarthritis. While I would like to see more evidence, ideally with longer-term studies, I would not discourage someone with osteoarthritis from considering turmeric, particularly if they are already following physical activity guidelines and really want to avoid over-the-counter analgesics. It is important to note that turmeric is not Advil however – there is no evidence you can pop two capsules and expect rapid, short-term pain relief. But the evidence for turmeric is encouraging, and with luck we will see additional trials in the future that confirm or refute these findings.

