Phopholipids are an essential component of cell membranes but their role in treating PMS is uncertain
A cure for PMS that is safe, natural, and proven effective by randomized, placebo-controlled, double-blind scientific trials? If only that were true!
Lipogen PMS-Free is a dietary phospholipid supplement containing PAS, a complex of 400 mg phosphatidylserine and 400 mg phosphatidic acid. It is described as “a nutritional supplement designed and clinically proven to alleviate the diverse symptoms of premenstrual syndrome (PMS).” Health News Review gave the press release a rating of only one out of five stars and described it as “all hat, no cattle.”
There’s a study!
The claim that Lipogen PMS-Free will reduce PMS symptoms has been tested in a randomized, placebo-controlled, double-blind clinical trial. The full text of that trial is freely available on the internet, but instead of linking to it, the Lipogen website asks readers to request a downloadable copy. They require customers to provide their contact information, which they then use to flood inboxes with emails that make several claims for the product and include a “Buy now” button.
The emails claim that the product:
- Reduces emotional symptoms
- Reduces depression
- Boosts mood
- Levels out mood swings
- Improves quality of life
- Reduces appetite problems
- Relieves sleep disturbances
- Lessens relationship interference
- Boosts cognitive performance
- Lowers impact on productivity
- Heightens concentration
- Improves functioning
But the study doesn’t support those claims.
What did the study actually show?
The rationale for doing the study was a word-of-mouth advertising campaign by the manufacturer in which 23 out of 220 women reported improvement in PMS. And they relied on findings of preclinical hormone studies suggesting there might be a role for PAS in improving memory, learning, mood, and stress management. Those studies had inconsistent results, and most were not really pertinent. A couple of examples: one study showed a lack of consistent evidence for cortisol dysregulation in premenstrual syndrome/premenstrual dysphoric disorder, and another tested the stress reactivity of chronically stressed male subjects.
The subjects were 40 women in Germany who had been diagnosed with PMS by a gynecologist. They did not take the actual product Lipogen PMS-Free, but took four doses a day of a pill containing 100 mg PS and 100 mg PA. They were followed through one baseline menstrual cycle and 3 treatment cycles. Various hormone tests were done (they called them biomarkers, but I don’t think that is accurate), and the subjects filled out a subjective daily record of symptoms.
Statistical analysis showed a significantly greater overall improvement in PMS symptoms compared to placebo (p = 0.001). That may seem impressive, but the graph is less convincing:
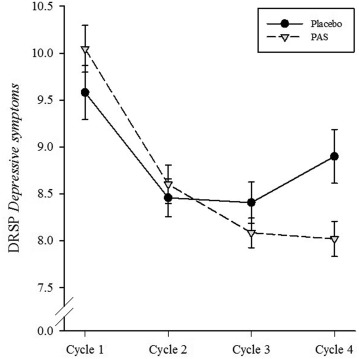
And the devil is in the details. The effects were small in magnitude. Many subscale results were negative and others only reached “a marginal level” of statistical significance, varying from p = 0.052 to p = 0.838. And there was no good evidence of actual clinical significance. For instance, the reduction of reported depressive symptoms was greater for the PAS group, but only at the level of p = 0.068. In other words, other than the overall score, the results of this study were negative, but the authors tried to cast them in a positive light. They concluded, “the positive results of this clinical study merits consideration of developing the PAS complex as a botanical drug for treatment of PMDD.” I don’t think that conclusion is justified by the data.
For what it’s worth, the study was financed by Lipogen Ltd, and one of the authors disclosed that he had “a commercial interest” in the product. In fact, he is the CEO of the Lipogen company. Not a reason to reject the study, but a reason for careful scrutiny.
Lipogen PS Plus
That same author (D.R.) was co-author of another study that can be downloaded from the company website for a related product, Lipogen PS Plus. “Positive Effects of Soy Lecithin-Derived Phosphatidylserine plus Phosphatidic Acid on Memory, Cognition, Daily Functioning, and Mood in Elderly Patients with Alzheimer’s Disease and Dementia.” As before, there is no reason to require a download and solicit identifying information, since the full text of the article is freely available on the web.
That article reports previously unpublished, early pilot studies, not of the product currently being sold, but of three daily doses of a pill containing 100 mg PS and 80 mg PA. They mention another study using six daily doses that failed to show any cognitive improvement. One study reported “positive” results that were actually not statistically significant (p = 0.84). Overall, this review of unpublished studies covered what were only pilot studies, with encouraging trends but requiring further study.
Another Lipogen product, Lipogen PSPA is advertised to help you stay calm and focused under stress. It was tested in 50 healthy male volunteers subjected to a stress test. It was apparently successful in normalizing salivary hormone levels. I didn’t bother to download that study; I could see where this was going and I didn’t want to waste any more time.
A pattern emerges
Lipogen would like you to believe that doctors have specifically designed an effective “natural” treatment for PMS. In fact, they already had a phospholipid supplement that they were marketing as a nootropic, and they merely repurposed it to fill another niche in the market. What Steven Novella wrote about nootropics was not encouraging. None of them have been proven effective. The idea is to find an aspect of brain function like memory, identify a metabolic precursor, and offer a nutritional supplement to supply that precursor. They tend to extrapolate from preclinical and pilot studies and make hyped-up speculative claims about presumed effectiveness that are not supported by good science. The studies cited often use a different dosage of the active ingredient, and do not represent typical use of the marketed product by a typical consumer. Positive results are often marginal. The researchers spin negative findings as positive findings that just didn’t quite reach statistical significance. They usually conclude that more study is needed, and this is certainly true.
Better studies needed
At least they understand the importance of randomized, placebo-controlled, double-blind studies, while so many CAM proponents don’t. That’s a step in the right direction. But here’s what I would like to see:
- Large, well-designed placebo-controlled studies of the actual products they are selling, with subjects typical of prospective customers
- Objective data to confirm subjective reports
- Independent replications of positive studies
- Reporting of confidence intervals
- Exit polls to check whether subjects could tell which group they were in
- Comparisons with other treatments that have been proven effective
- Discussion of contraindications and reported adverse effects
Bottom line
Phospholipid supplements may be effective as PMS remedies and/or as nootropics, but so far the evidence is far from compelling.

