It is a triumph of marketing over evidence that so many people believe dietary supplements are natural, safe, and effective. Supplements actually cause a significant amount of harm, and are associated with at least 23,000 emergency department visits and 2,000 hospitalizations per year. Supplements are consumed by 50% of Americans and incredibly, 70% of United States military service members. As they are widely sold and are subject to weak regulation, the routine collection of evidence and reports of the harms of supplements is hard to come by. The FDA relies on passive reporting, including collecting adverse event reports and consumer complaints, inspecting manufacturing facilities, and monitoring imported ingredients. In exceptional circumstances, the FDA can take supplements off the market by issuing a recall. In a new survey, over 26,000 US service members provide reports on adverse effects of dietary supplements. The report highlights which supplements are associated with the most adverse effects.
Evidence of harms
Dietary supplements encompass an array of products that include vitamins, minerals, herbal remedies, and other products. As I’ve discussed over the past several years here at the blog (and also discussed by others), there is little assurance provided by regulators that consumers can trust that what’s on the label of a dietary supplement is actually in the bottle. Quality standards are not enforced in the same way as for prescription or over-the-counter drugs, and consequently there are greater risk of contamination or dose variation owing to a weak regulatory framework that doesn’t mandate review before a product can be sold. Even if the label is 100% accurate, and the dose itself is standardized, there is also nothing intrinsic to a dietary supplement or natural product that it cannot cause harm in some way. If a substance can have beneficial effects, then it can have negative effects. You may recall that the amphetamine-line 1,3–dimethylamylamine (DMAA) was a popular dietary supplement that had the rare but unfortunate side effect of death. It was eventually forced from the market by the FDA.
This paper discusses the US Military Dietary Supplement Use Study which was a random sample of military service members who completed a survey on supplement use and adverse effects experienced. Recruitment began in 2018 and surveys continued through August 2019. The questionnaire listed 96 generic dietary supplements (e.g., multivitamins, individual vitamins, proteins/amino acids, herbals products) as well as 67 named products. The branded products were identified by looking at dietary supplements sales directly to service members (via the military’s Exchange service) and also by surveying General Nutrition Centre stores on or near military installations. There were also open text fields where supplements not otherwise listed could be added.
The survey asked service members if they had used a particular dietary supplement in the past six months and if they believed they had experienced a particular “side effect” from using that supplement. A list of side effects were presented (e.g., palpitations, abdominal pain, nausea/vomiting, etc.) as well an “other” category with the opportunity to describe the effect.
The 96+ dietary supplements were grouped into the following categories:
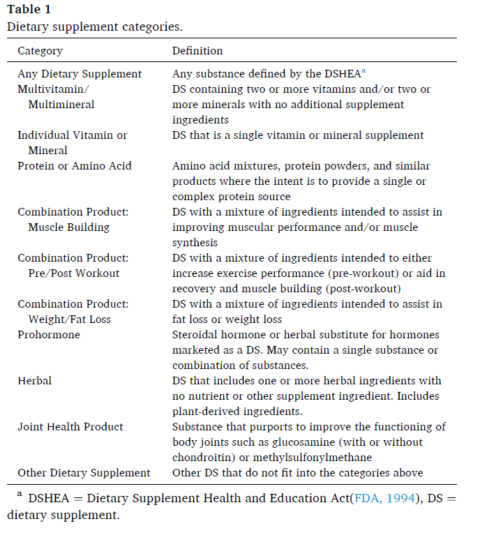
Table 1. Dietary supplement categories.
For each dietary supplement and for each category, the total number of users and the number of users reporting at least one adverse event where counted. The proportion of users reporting adverse events were then calculated, along with the standard error. For dietary supplements associated with at least 25% of user reporting adverse events, each individual event was also compiled with frequency and standard error.
Results
Of the 200,000 service members identified, 73% were contacted successfully, and 126,681 (18.2%) signed the consent and completed the survey. Here are the proportion of service members reports that included at least one adverse event.

The combination product category (which included body building, fat/weight loss and pre/post workout products) had the highest adverse event prevalence. Joint health products had the least. Additional tables show the adverse event for individual ingredients and products. Vitamins were generally well tolerated, either as multivitamins or individual ingredient products. Some branded multivitamins were associated with greater reports of adverse events. Not surprisingly, niacin, which can cause face flushing, was the poorest-tolerated individual vitamin.
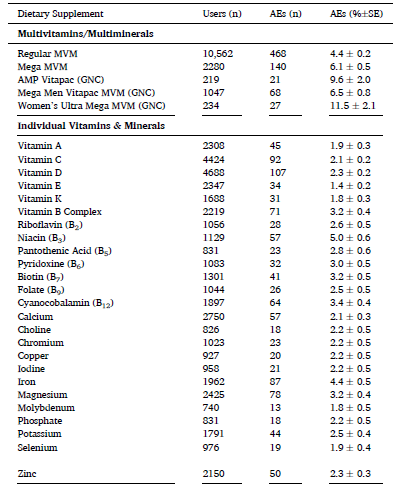
Products marketed for weight and fat loss were associated with considerable side effects:
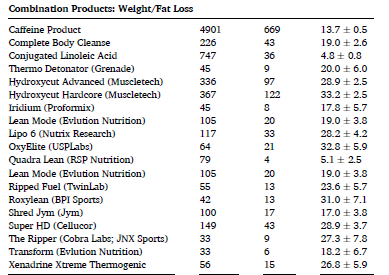
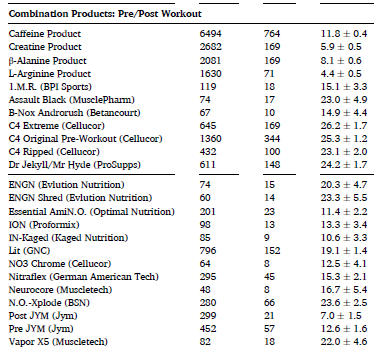
The same can be said for pre/post workout supplements (below) as well as products sold as “prohormones”:
Turning to herbals and remedies for “joint health”, side effects were reported for most products, at varying degrees of frequency:

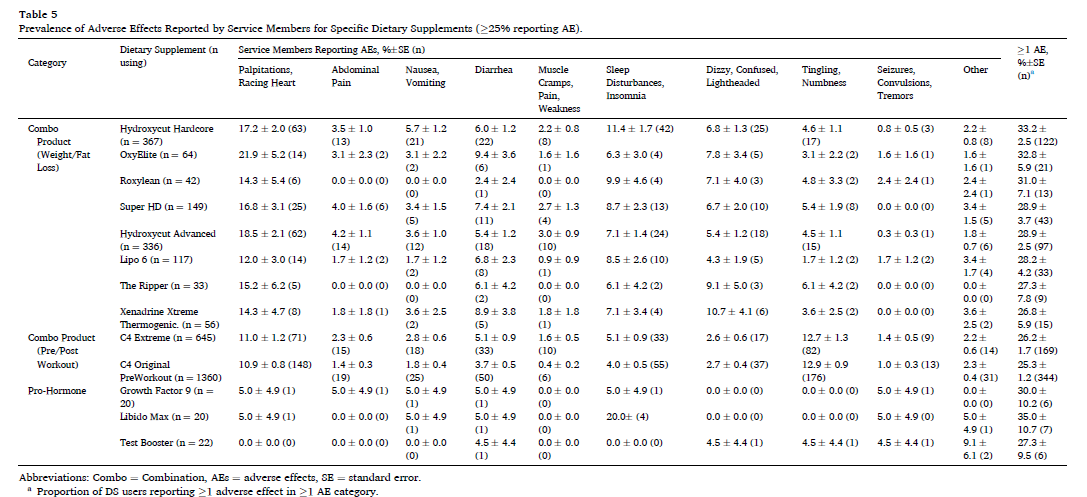
Finally, here is the prevalence of specific adverse effects reported for specific products. The reported rates of heart palpitations are quite high with some of these products.
Conclusion: More evidence against supplement safety
In this large sample of US service members, the overall proportion of reported adverse effects was generally low, with notable exceptions in some categories and with some products. Over 10% of users reported adverse effects for 89% of weight loss products, 83% of pre/post workout products, and 53% of “prohormone” products. Adverse effects were most commonly reported with combination (“muscle building”) products and related products also had high adverse effect report rates. This could be due to the ingredients in these products or possibly a consequence of multi-ingredient supplements causing additive side effects.
There were several limitations to this trial. This was self-reported data, based on recall. It could have potentially biased toward those that do associate adverse effects with supplements, as those not identifying any effects may not have been as willing to do the survey. The survey did not measure dosages, so we do not know if the products were being used “as labelled” or some other way. Because not every dietary supplement could be listed, it is possible the products were missed. It was also not possible to identify users taking multiple dietary supplements at one time, and determine how adverse effects were associated with a particular product. This survey did not look at the medical consequences of the reported adverse effects.
This survey provides new information to show that specific categories of dietary supplements (weight loss, pre/post workout, and body building products and prohormones) are more likely than other supplements to be associated with adverse effects. Consumers that want to reduce their risk of adverse effects from dietary supplements may want to avoid these categories of products.